
RESEARCH PAPERS
Clinical & Experimental Dermatology Research Article Morgellons Disease:
A Chemical and Light Microscopic Study
Marianne J. Middelveen 1, Elizabeth H. Rasmussen 2, Douglas G. Kahn 3 and Raphael B. Stricker
1* 1 International Lyme and Associated Diseases Society, Bethesda, MD 2 College of Health Sciences, University of Wyoming, Laramie, WY 3 Department of Pathology, Olive View - UCLA Medical Center, Sylmar, California
http://www.omicsonline.org/2155-9554/2155-9554-3-140.pdf
Abstract
Morgellons disease is an emerging multisystem illness characterized by unexplained dermopathy and unusual skin associated filament production. Despite evidence demonstrating that an infectious process is involved and that lesions are not self-inflicted, many medical practitioners continue to claim that this illness is delusional. We present relevant clinical observations combined with chemical and light microscopic studies of material collected from three patients with Morgellons disease. Our study demonstrates that Morgellons disease is not delusional and that skin lesions with unusual fibers are not self-inflicted or psychogenic. We provide chemical, light microscopic and immunohistological evidence that filaments associated with this condition originate from human epithelial cells, supporting the hypothesis that the fibers are composed of keratin and are products of keratinocytes.
Keywords:
Morgellons disease; Digital dermatitis; Lyme disease;
Borrelia burgdorferi; Spirochetes; Keratin
Introduction
Morgellons disease (MD) is an emerging dermatological disorder and multisystem illness. The disease is characterized by unexplained dermopathy associated with formation of unusual filaments found both subcutaneously and emerging from spontaneously appearing, slow-healing skin lesions [1]. Filaments associated with MD appear beneath unbroken skin [1,2], thus demonstrating that they are not self-implanted.
Filaments have been observed protruding from and attached to a matrix of epithelial cells [3]. This finding demonstrates that the filaments are of human cellular origin and are not textile fibers.
These filaments have not been matched with known textile fibers, and dye-extracting solvents have failed to release coloration; the fibers are also very strong and heat resistant [4,5]. MD filaments are physically and chemically consistent with keratin, a biofiber produced in the epithelium by keratinocytes. A recent report from the Centers for Disease Control and Prevention (CDC) confirmed that these filaments have a protein composition that is consistent with keratin [6].
Lyme disease-like symptoms in MD such as neurological disorders and joint pain are evidence of systemic involvement [1,2,7]. Objective clinical evidence of disease has been demonstrated by its association with peripheral neuropathy, delayed capillary refill, decreased body temperature, tachycardia, elevated pro-inflammatory markers, cytokine release, selective immune deficiency and elevated insulin levels, suggesting that an infectious process is involved [8,9]. Patients may demonstrate abnormal laboratory findings indicative of low-grade anemia, endocrine dysfunction, immune dysfunction and inflammation [8,10]. Patients with MD are predominantly sero-reactive to Borrelia
burgdorferi (Bb) antigens, suggesting a likelihood of Lyme borreliosis or related spirochetal infection [1,10]. Patients also demonstrate a higher than expected percentage of positive laboratory findings for other tick-borne diseases, suggesting the possible involvement of coinfecting pathogens [10].
The observation of unusual filaments forming in lesions is not unique to humans afflicted with MD. Similarities between MD and bovine digital dermatitis (BDD) have been described [3]. BDD is an emerging disease afflicting cattle and is characteristically associated with unusual filament formation in skin above the hooves [11]. Latestage proliferative lesions demonstrate elongation of keratinocytes, hyperkeratosis, and proliferation of long keratin filaments [12-14]. Consistent detection of spirochetes associated with lesions is evidence of spirochetal etiologic involvement [15-20]. Experimental induction of lesions with tissue homogenates [21] and pure cultured treponemes [22] supports a role for spirochetes as primary etiologic agents.
Like BDD, MD is associated with apparent spirochetal infection and unusual filament production [3]. A comparison between BDD and MD suggests that the unusual fibers seen in MD patients may result from hyperkeratosis and filament production as described in BDD. It appears that MD fibers are likewise composed of keratin produced by
keratinocytes, a phenomenon that has been demonstrated in BDD [3]. The following three case studies provide further evidence supporting this hypothesis.
Materials and Methods
Human and bovine samples
Three patients meeting the clinical criteria for Morgellons disease collected calluses, scabs, filaments, and other dermatological debris and submitted the material for microscopic examination. The collected samples were examined by bright-field microscopy at 100x magnification. Specimens were illuminated either superior or posterior to the specimen. Some specimens were also illuminated with ultraviolet (UV) light.
Biopsies from cattle with BDD were kindly provided by Dr. Dorte Dopfer, Faculty of Veterinary Medicine, University of Wisconsin, Madison, WI. Biopsy material from proliferative late stage BDD was examined for comparison to MD samples with 8x magnification under a dissecting microscope. This material was also tested for fluorescence under UV light.
For the chemical experiments, samples of normal hair, filaments from Cases 1 and 2 and BDD fibers were studied for reactivity to three caustic agents: sodium hypochlorite 12%, sodium hydroxide 10%, and potassium hydroxide 10%. Each sample was suspended in 150 µl of the chemical solution for up to two hours, and serial light microscopy was
performed at 0, 1, 10, 30, 60 and 120 minutes. Dissolution of fibers was assessed by fraying, loss of shape and/or disintegration at each timepoint.
For the immunohistological experiments, filament samples from Cases 1 and 2 were stained for keratin using monoclonal antibodies. Briefly, formalin-fixed paraffin-embedded filaments were incubated with monoclonal antibodies AE1/AE3 (Dako North America Inc, Carpinteria, CA) and AE5/AE6 (Cell Marque Corporation, Rocklin, CA) directed against cytokeratins 1/3 and 5/6, respectively, using the Envision® + Dual-Link System-HRP (Dako) according to the manufacturer’s instructions. The samples were stained using a horseradish peroxidase label, and the brown staining of keratin was visualized under light microscopy
Clinical Observations
Case 1
The patient is a 72-year-old grandmother and former fashion model who developed painful lesions on her hands while working in her garden in San Antonio, Texas, in 1994.
The lesions were punctate with ragged edges and healed slowly, leaving visible scarring. Fibers were observed in the lesions and under intact skin on her hands using a 60x handheld microscope. Topical steroids had no effect. The patient also noted the onset of fatigue, joint pain and muscle aches, and systemic steroid treatment exacerbated these symptoms without any improvement in the skin lesions. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation was entirely normal. Biopsy of a lesion demonstrated hyperkeratosis and parakeratosis with no visible organisms or evidence of vasculitis. However “textile fibers” were noted in the dermal layer of the biopsy specimen.
In 2001, after numerous visits to dermatologists and other medical specialists and treatment with topical emollients and antiinflammatory medications, the patient had persistent skin lesions on her hands, fatigue and musculoskeletal pain. Despite the use of gloves to avoid scratching, her lesions persisted and she was unable to work in her garden or hold her grandchildren due to pain in her hands and joints. She recalled numerous tickbites but never saw an erythema migrans (EM) rash, and she was found to have positive testing for B. burgdorferi, Babesia microti and Bartonella henselae. She was treated with antimicrobial medications and her fatigue and musculoskeletal pain improved significantly. However her skin lesions persisted. She received anti-parasitic medication, and the lesions improved to the point that she could once again do gardening. The lesions persist but are “manageable” (Figure 1A).
Case 2
The patient is a 49-year-old registered nurse who had numerous tickbites while hiking, camping and horseback riding in Missouri, Texas and Northern California over more than a decade. She never saw an EM rash. In 1997 while living in San Francisco she eveloped painful lesions on her face, trunk and extremities. The lesions were punctate with ragged edges. Some lesions healed slowly, leaving visible scarring, while others did not heal at all, and fibers that were resistant to extraction were observed within several lesions. Fibers were also observed under intact skin using a 60x handheld microscope.
Topical steroids had no effect. Biopsy of a lesion on her leg revealed hyperkeratosis and parakeratosis without evidence of infection or vasculitis. However, “textile fibers” were noted in the dermal layer of the biopsy specimen. She also developed fatigue and musculoskeletal pain, and systemic steroid treatment exacerbated these symptoms without any improvement in the skin lesions. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation was completely normal.
Because of persistent fatigue, musculoskeletal pain and her history of tick exposure, the patient was evaluated for Lyme disease in 2004 and had positive testing for B. burgdorferi and Ehrlichia chafeensis. Antibiotic therapy led to improvement in the fatigue and musculoskeletal pain, but the skin lesions persisted. She received antiparasitic medication and her skin lesions improved somewhat, but new lesions appeared and healing lesions caused painful scarring. She has received intermittent courses of antibiotics over the past six years, and her skin lesions continue to wax and wane.
Case 3
The patient is a 47-year-old business manager who was in excellent health until he developed a “bullseye” rash, fever, chills, severe headache, musculoskeletal pain and malaise after hiking in the woods near Atlanta, Georgia, in 1995. He had pulled ticks off his dog, which also became ill at the same time. He was diagnosed with fibromyalgia and treated with pain medications, but by 2000 he had become progressively disabled by muscle pain and fatigue.
In 2002 he developed crawling sensations on his head, face, groin and other body areas where there was hair. The sensations were accompanied by painful skin lesions. He was diagnosed with folliculitis and put on a topical antibiotic, which made his skin symptoms worse. He began to notice painful fibers coming out of the skin on his face, head and other hirsute areas, and he could not sleep because the fibers were so painful.
He extracted fibers from his facial lesions, but new ones appeared. He was diagnosed with trichotillomania and delusional parasitosis.
He went to several dermatologists and was treated with topical lindane and oral cephalexin without benefit. Treatment with oral ketoconazole and fluconazole provided marginal improvement in the crawling sensations and skin lesions. A scalp biopsy demonstrated increased numbers of catagen and telogen follicles with fragmented hair fibers and inner root sheath consistent with trichotillomania.
There were no visible organisms or evidence of vasculitis. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation revealed reactive depression. He was treated with antidepressants without benefit.
Finally in 2005 a physician noted fibers under his skin using a 60x hand-held microscope.
Testing for Lyme disease was indeterminate in 2006, and treatment with doxycycline was given for one month without benefit. The patient continues to suffer from crawling sensations, skin lesions, musculoskeletal pain, disabling fatigue and depression. He is reluctant to see any more physicians about his skin condition (Figure 1C).
Results
MD Microscopic observations
Case 1: Microscopic examination revealed a wide range of filaments in various stages of formation ranging from early stages that demonstrated either single or clusters of hyaline, tentacle-like projections with tapered ends (tentacle diameter approximately 5 µm) to macroscopic masses or mats of tangled fibers (approximately 1 mm diameter). Floral-like formations of early-stage filaments were observed in some samples that were collected on different dates and years. These structures had tapered ends with bases originating at a central point and were found in groups anchored to a dried dermal matrix. The reverse side of some of these specimens revealed a layer of pavement epithelial cells. Epithelial matrices anchoring longer hyaline fibers were observed, suggesting that as the tentacle-like projections increase in length individual fibers may become tangled, or clumped. Various structures composed of clumps, strings, and nest-like balls of hyaline filaments were observed and some of these were glued together by clotted or dried exudate. This suggests that tangled filaments may eventually separate from the supporting epithelial matrix and form balls and other tangled structures.
Some samples revealed raised unidentified papules protruding from dried epithelial tissue that might be abnormal hair follicles. Long isolated colored filaments, filament fragments, balls, and clumps of fibers (red, blue, black and green) were also observed, but were not attached to or growing from epithelial tissue. Many of these colored filaments had bulb-like ends (50 µm diameter) that looked very much like those found in hair follicles.
Many fibers displayed iridescence under bright-field microscopy and were fluorescent under UV lighting. Hyaline or white fibers fluoresced brightly, as did blue fibers. Red and green fibers displayed striking iridescence but fluoresced with less intensity than the blue and white fibers. This suggests that melanin pigments may be associated with red and green filaments.
Early floral-shaped clusters were brightly fluorescent. Human hair was not fluorescent nor was normal skin. Color intensity and hue of the red and blue filaments was influenced by the color spectrum of the illuminating light. This property and the presence of iridescence suggests that a structural component is involved in the unusual colors
seen in Morgellons fibers.
Case 2: Microscopic examination of scab material revealed scab detritus imbedded with long filaments of various colors. Hyaline, red, blue, and light purple fibers were observed (10-40 µm diameter). One sample revealed fibers tangled around a hair and these fibers may have been associated with the hair follicle. Smaller, pale purple fibers (10 µm diameter) appeared to form a mesh around the follicle. Some samples revealed fibers that lay beneath or penetrated dermal tissue.
Case 3: Microscopic examination was performed with particular attention to hair follicles, as the patient had reported unusual filament formation associated with the follicles. Microscopy revealed abnormalities of the follicular bulbs and the hair associated with these follicles that indicated abnormal functioning of follicular keratinocytes.
Many follicles contained malformed bulbs with distorted shapes, and some follicles had two or more hairs branching from a single inner root sheath. Filaments stemming from the bulb end were found in some follicles and these appeared as rootlike growths. Transparent filaments were observed that stemmed from cells within the inner root sheath.
On some hairs red or blue colored filaments branched from the shaft. Many hairs were flattened or tape-like on cross-section rather than concentric. These hairs were similar in appearance to Morgellons filaments.
BDD Microscopic observations Biopsies from late proliferative stage BDD lesions were examined microscopically for comparison. Although the scale of filaments was much larger, the BDD filaments (roughly ten times larger) were similar in appearance compared to the specimens observed in Case 1. Filaments were macroscopic, opaque and dirty white in color, ranging in size from less than 0.5 mm in diameter to about 1 mm in diameter. In cross section filaments appeared to originate beneath the stratum corneum. Longer filaments were close to 1 mm in length. The BDD filaments demonstrated fluorescence under UV light.
Chemical Experiments
Samples of normal hair, colored filaments and dermal material from Cases 1 and 2, and BDD fibers were subjected to immersion in caustic agents. Duplicate experiments with each caustic agent were performed on each sample. Results of the experiments are shown in (Table 1) Normal hair and patient filaments began to fray after incubation for 1 minute, and the patient filaments had completely disintegrated after incubation for 120 minutes in 12% sodium hypochlorite. Normal hair was still visible at this timepoint. In contrast, patient filaments began to fray at 1 minute in 10% sodium hydroxide but were still visible after 120 minutes, similar to normal hair. The hair and patient filaments
were more resistant to 10% potassium hydroxide, with visible fraying beginning at 10 minutes and fibers still visible at 120 minutes. Although the larger BDD fibers appeared to be more resistant to the chemicals, fraying and shape change similar to the human samples was evident at 120 minutes with each caustic agent.
Keratin immunostaining
The results of keratin immunostaining experiments are shown. The MD filaments from Case 1 stained strongly with the “pankeratin” antibody AE1/AE3 directed against cytokeratin 1/3.
In contrast, the filaments stained weakly with the more restrictive antibody AE5/AE6 directed against cytokeratin 5/6. Staining with AE1/AE3 was seen over the length of the fiber, while staining with AE5/ AE6 was only detected in the outermost scale. Melanin pigmentation
was not seen in the fibers. No staining was detected with an irrelevant monoclonal antibody, and similar positive keratin staining with AE1/AE3 was detected in MD fibers from Case 2 (data not shown).
Discussion
Our three patients had features of MD that are commonly described in the medical literature, including insidious onset, dermatological signs and systemic symptoms, lack of response to immunosuppressive treatment and association with tickborne diseases [1-3].
Case 1 had skin lesions confined to the hands, while Cases 2 and 3 had disseminated skin lesions over the head, trunk and extremities. In addition, Case 3 had symptoms associated primarily with hair follicles, and a sensation of change in hair composition and texture is often reported by Morgellons patients [1,10]. These MD patterns have been recognized in prior studies [1,2] and we propose a classification of localized MD versus disseminated MD based on the distribution of the dermopathy. Although the reason for this dermopathy distribution is unknown, the location of skin lesions may be related to the cell of origin of the fibers seen in lesions or under the skin, as discussed below. Further study of the dermopathy distribution in MD is warranted.
The present study demonstrates Morgellons filaments that clearly originate from a layer of pavement epithelial cells visibly held together by desmosomes. The predominant cells found in pavement epithelial tissue are keratinocytes. We also noted MD fibers that clearly originate from the inner root sheaths of hair follicles, and keratinocytes are the predominant cell type in this tissue. Keratinocytes produce the biofiber keratin. A cross section of BDD filaments likewise demonstrates filament origin from cells beneath the stratum corneum, consistent with descriptions in the literature of growth from keratinocytes [14,19]. Thus MD filaments and BDD filaments appear to be similar in formation at the cellular level, both originating from keratinocytes in the stratum spinosum or stratum basale. MD differs from BDD, however, in that MD filaments appear to originate from follicular keratinocytes as well as epidermal keratinocytes. Both MD filaments and BDD filaments fluoresce in UV light. We have also shown for the first time that MD filaments contain keratin, and keratin staining was positive using a “pankeratin” monoclonal antibody but negative with a more restricted keratin ligand. This observation indicates that the fibers originate from specific tissues that require further characterization.
The observation that MD fibers are found beneath unbroken skin, may grow from an epidermal matrix and are associated with hair follicles suggests that they are not self-implanted textile fibers [1-3]. The filament formation described in MD is associated with a high likelihood of Bb infection [1,10]. BDD in cattle is associated with hyperkeratosis, keratin filament formation and spirochetal infection [12-20]. Hyperkeratosis and excessive keratin production associated with chronic inflammation has been demonstrated in humans with cholesteatoma [23,24], and alterations in keratinocyte expression of HLA markers and tissue enzymes have been reported in association with Bb infection [25,26]. These observations suggest that hyperkeratosis and keratin filament production associated with spirochetal infection is a plausible explanation for the clinical and microscopic findings in
MD.
Hyaline and colored filaments from the three case studies demonstrate iridescence and an appearance consistent with keratin. Red, blue, purple and black are colors found in keratin and are associated with structural coloring and/or melanin production [27-30]. Clusters of early filaments described in Case 1 demonstrate that fibers are anchored and growing from a basal epithelial cell matrix.
They are clearly biological and human in nature and are not implanted textile fibers. Various growth stages of fibers attached to epidermal matrices have been observed. These range from early filaments isolated or in clusters (that are only a few µm in diameter and 10 µm long) to long tangled mats (with fibers 10 µm or wider in diameter and several hundred µm long). Similar filament structures have previously been reported and photographed in MD [31]. Textile fibers have never been produced in this manner, and the suggestion that these unusual formations are manufactured textile fibers is not credible.
Longer fibers with tapered ends anchored to a cellular matrix were observed in Case 1, demonstrating filament evolution. Colored fibers were often found near larger hair follicles or appeared to have follicular bulb-like ends, suggesting an association with hair follicles and follicular keratinocytes. Our chemical studies suggest that MD filaments and
BDD fibers react to caustic agents in a manner similar to normal hair, although MD filaments appeared to be more susceptible and BDD fibers less susceptible to the caustic agents.
In preliminary studies using scanning electron microscopy, the presence of scales on a blue filament indicated that this specimen was a fine hair (D’Alba L and Shawkey MD, unpublished observation, December 2011). This finding suggests that some of the colored fibers of follicular origin may in fact be modified hairs. Differences between the keratinocytes found in the inner root sheath of hair follicles and keratinocytes found in the basal skin layer may account for the differences of location, structure, coloring and size of fibers seen in this study [32,33]. The effect of spirochetes on keratinocyte function may also play a role in altered keratin production associated with MD and BDD [22,25,26].
In conclusion, MD lesions were not caused by self-mutilation or delusions in the three cases presented here. The photographic evidence clearly demonstrates that the unusual fibers or filaments described in this study are not self-implanted textile fibers. All three patients had symptoms and laboratory findings consistent with systemic illness and
indicative of tickborne disease. Neuropsychiatric testing was normal in two cases and influenced by the disease in the third case, and all three patients were examined by a medical practitioner who confirmed the presence of fibers underneath unbroken skin compatible with a diagnosis of MD.
We have demonstrated that filaments found in MD patients have chemical, physical and immunohistological features of keratin. The presence of individual filaments attached to epithelial tissue is consistent with keratin and suggests that the filaments are produced by keratinocytes. Morgellons filaments have been photographed growing from pavement epithelial cells, and this process resembles the evolution of filaments seen in BDD.
Because BDD is a disease in which spirochetes have been identified as primary etiologic agents, and spirochetal sero-reactivity has been associated with MD, it is reasonable to assume that spirochetal infection plays an important role in MD filament production. Further immunohistological and electron microscopy studies are needed to solve the mystery of Morgellons filaments.
Conflict of Interest Statement
RBS serves without compensation on the medical advisory panel for QMedRx
Inc. He has no financial ties to the company. MJM, EHR and RBS serve without
compensation on the scientific advisory panel of the Charles E. Holman Foundation.
DGK has no conflicts to declare.
Acknowledgments
The authors thank Drs. Gordon Atkins, Robert Bransfield, Dorte Dopfer,
Alan MacDonald, Peter Mayne, Deryck Read, Matthew Shawkey, Janet Sperling,
Ginger Savely, Michael Sweeney and Randy Wymore for helpful discussion. We
thank Dr. Robert B. Allan for technical support and Lorraine Johnson for manuscript
review, and we are grateful to Harriet Bishop, Cindy Casey and Lee Laskowsky for
providing first-hand information about Morgellons disease.
References
1. Savely VR, Leitao MM, Stricker RB (2006) The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol 7: 1-5.
2. Savely VR, Leitao MM (2005) Skin lesions and crawling sensations: disease or delusion? Adv Nurse Pract 13: 16-17.
3. Middelveen MJ, Stricker RB (2011) Filament formation associated with spirochetal infection: A comparative approach to Morgellons disease. Clin Cosmet Investig Dermatol 4: 167-177.
4. Elkan D. Morgellons (2007) disease the itch that won’t be scratched. New Scientist 2621: 46-49.
5. Wymore RS (2011) Morgellons disease research; shotgun DNA analysis, PCR, microscopy and spectroscopy. Morgellons Medical Conference, Austin, Texas.
6. Pearson ML, Selby JV, Katz KA, Cantrell V, Braden CR, et al. (2012) Clinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathy. PLoS ONE 7: e29908.
7. Savely VR, Stricker RB (2007) Morgellons disease: the mystery unfolds. Expert Rev Dermatol 2: 585-591.
8. Harvey WT (2007) Morgellons disease. J Am Acad Dermatol 56: 705-706.
9. Harvey WT, Bransfield RC, Mercer DE, Wright AJ, Ricchi RM, et al. Morgellons disease, illuminating an undefined illness: a case series. J Med Case Reports 3: 8243.
10. Savely VR, Stricker RB (2009) Morgellons disease: analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiology. Clin Cosmet Investig Dermatol 3: 67-78.
11. Cheli R, Mortellaro CM (1974) Digital dermatitis in cattle. Proc 8th Int Meet Dis Cattle, Milan, Italy 8: 208-213.
12. Vink WD, Jones G, Johnson WO, Brown J, Demirkan I, et al. ( 2009) Diagnostic assessment without cut-offs: application of serology for the modeling of bovine digital dermatitis infection. Prev Vet Med 92: 235-248.
13. Dopfer D, Willemann MA(1998) Standardisation of infectious claw diseases. Proceedings of the 10th International Symposium on Lameness in Ruminants; Lucerne, Switzerland 244-254.
14. Borgmann JE, Bailey J, Clark EG (1996) Spirochete-associated bovine digital dermatitis. Can Vet J 37: 35-37.
15. 15. Blowey RW, Sharp MW (1988) Digital dermatitis in dairy cattle. Vet Rec 122: 505-508.
16. Read DH, Walker RL, Castro AE, Sundberg JP, Thurmond MC (1992) An invasive spirochaete associated with interdigital papillomatosis of dairy cattle. Vet Rec 130: 59-60.
17. Scavia G, Sironi G, Mortellaro CM, Romusi S (1994) Digital dermatitis: further contribution on clinical and pathological aspects in some herds in northern Italy. Proc Int Symp Dis Ruminant Digit 8: 174-176.
18. Grund S, Nattermann H, Horsch F (1995) Electron microscopic detection of spirochetes in dermatitis digitalis of cattle. Zentralbl Veterinarmed B 42: 533-542.
19. Döpfer D, Koopmans A, Meijer FA, Szakáll I, Schukken YH et al. ( 1997) Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet Rec 140: 620-623.
20. Demirkan I, Carter SD, Murray RD, Blowey RW, Woodward MJ(1998) The frequent detection of a treponeme in bovine digital dermatitis by
immunochemistry and polymerase chain reaction. Vet Microbiol 60: 285-292.
21. Read DH, Walker RL(1998) Papillomatous digital dermatitis (footwarts) in California dairy cattle: clinical and gross pathologic findings. J Vet Diagn Invest 10: 67-76.
22. Gomez A, Cook N, Dopfer D, Bernardoni N, Dusick A, et al. (2011) An experimental infection model for digital dermatitis. Lameness in Ruminants Conference, New Zealand.
23. Hamajima Y, Komori M, Preciado DA, Choo DI, Morobe K et al. (2010) The role of inhibitor of DNA-binding (ID1) in hyperproliferation of keratinocytes: the pathological basis for middle ear cholesteatoma from chronic otitis media. Cell Prolif 43: 457-463.
24. Raynov AM, Choung YH, Park HY, Choi SJ, Park K (2004) Establishment and characterization of an in vitro model for cholesteatoma. Clin Exp
Otorhinolaryngol 1: 86-91.
25. Tjernlund U, Scheynius A, Asbrink E, Hovmark A (1986) Expression of HLA-DQ antigens on keratinocytes in Borrelia spirochete-induced skin lesions. Scand J Immunol 23: 383-388.
26. Gebbia JA, Coleman JL, Benach JL (2001) Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect Immun 69: 456-462.
27. Shawkey MD, Hill GE (2005) Caratenoids need structural colors to shine. Biol Lett 1: 121-124.
28. Shawkey MD, Shreekumar RP, Hill GE, Siefferman LM, Roberts SR (2007) Bacteria as an agent for change in structural plumage color: correlational and experimental evidence. Am Nat 169: S112-S121.
29. D’Alba L, Saranathan V, Clarke JA, Vinther JA, Prum RO, et al. (2011) Colourproducing ß-keratin nanofibres in blue penguin (Eudyptula minor) feathers. Biol Lett 7: 543-546. .
30. Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, et al. (2011) Biomimetic selftemplating supramolecular structures. Nature 478: 364-368.
31. Charles E. Holman Foundation (2012) Accessed February 16.
32. Rugg EL, Leigh IM (2004) The keratins and their disorders. Am J Med Genet C Semin Med Genet 131C: 4-11.
33. Moll R, Divo M, Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129: 705-733.
Filament formation associated with spirochetal infection:
a comparative approach to Morgellons disease
October 4, 2012 http://www.morgellons-uk.net/?p=824
Abstract
Bovine digital dermatitis is an emerging infectious disease that causes lameness, decreased milk production, and weight loss in livestock. Proliferative stages of bovine digital dermatitis demonstrate keratin filament formation in skin above the hooves in affected animals. The multifactorial etiology of digital dermatitis is not well understood, but spirochetes and other coinfecting microorganisms have been implicated in the pathogenesis of this veterinary illness.
Morgellons disease is an emerging human dermopathy characterized by the presence of filamentous fibers of undetermined composition, both in lesions and subdermally.
While the etiology of Morgellons disease is unknown, there is serological and clinical evidence linking this phenomenon to Lyme borreliosis and coinfecting tick-borne agents. Although the microscopy of Morgellons filaments has been described in the medical literature, the structure and pathogenesis of these fibers is poorly understood. In contrast, most microscopy of digital dermatitis has focused on associated pathogens and histology rather than the morphology of late-stage filamentous fibers.
Clinical, laboratory, and microscopic characteristics of these two diseases are compared.
Morgellons Disease: A Chemical and Light Microscopic Study
http://www.omicsonline.org/2155-9554/2155-9554-3-140.pdf
Morgellons disease, illuminating an undefined illness: a case series
http://www.biomedcentral.com/content/pdf/1752-1947-0003-0000008243.pdf
William T. Harvey 1*,
Robert C. Bransfield 2,
Dana E. Mercer 3,
Andrew J. Wright 4,
Rebecca M. Ricchi 5 and
Mary M Leitao 6
Addresses:
1 Preventive Medicine, Colorado Springs, CO 80949, USA,
2 Psychiatry, Red Bank, NJ, 07701, USA,
3 Veterinary Medicine, Fulton, TX, 78358, USA,
4 General Practitioner, Bolton BL1 4QR, UK,
5 Adult Medicine, USAFA, CO, 80840, USA and
6 BS (Biology), Guilderland, NY 12084, USA
Email:
WTH* - idmed99@aol.com
RCB - bransfield@comcast.net
DEM - drdana@horizonvetclinic.net
AJW - drajw2003@yahoo.co.uk;
RMR - rebecrn@comcast.net
MML - MaryL@Morgellons.org
* Corresponding author
Received: 28 November 2008
Accepted: 17 March 2009
Published: 1 July 2009 Journal of Medical Case Reports 2009, 3:8243 doi: 10.4076/1752-1947-3-8243
This article is available from: http://jmedicalcasereports.com/jmedicalcasereports/article/view/8243
© 2009 Harvey et al; licensee Cases Network Ltd.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract Introduction:
This review of 25 consecutive patients with Morgellons disease (MD) was undertaken for two primary and extremely fundamental reasons. For semantic accuracy, there is only one “proven” MD patient: the child first given that label. The remainder of inclusive individuals adopted the label based on related descriptions from 1544 through 1884, an internet description quoted from Sir Thomas Browne (1674), or was given the label by practitioners using similar sources.
Until now, there has been no formal characterization of MD from detailed examination of all body systems.
Our second purpose was to differentiate MD from Delusions of Parasitosis (DP), another “informal” label that fit most of our MD patients. How we defined and how we treated these patients depended literally on factual data that would determine outcome. How they were labeled in one sense was irrelevant, except for the confusing conflict rampant in the medical community, possibly significantly skewing treatment outcomes.
Case presentation:
Clinical information was collected from 25 of 30 consecutive self-defined patients with Morgellons disease consisting of laboratory data, medical history and physical examination findings. Abnormalities were quantified and grouped by system, then compared and summarized, but the numbers were too small for more complex mathematical analysis. The quantification of physical and laboratory abnormalities allowed at least the creation of a practical clinical boundary, separating probable Morgellons from non-Morgellons patients. All the 25 patients studied meet the most commonly used DP definitions.
Conclusions:
These data suggest Morgellons disease can be characterized as a physical human illness with an often-related delusional component in adults. All medical histories support that behavioral aberrancies onset only after physical symptoms. The identified abnormalities include both immune deficiency and chronic inflammatory markers that correlate strongly with immune cytokine excess.
The review of 251 current NLM DP references leads us to the possibility that Morgellons disease and DP are grossly truncated labels of the same illness but with the reversal of the cause-effect order. Further, the patients’ data suggest that both illnesses have an infectious origin.
Introduction
The term “Morgellons disease” first publicly appeared on the Internet in 2002. The “index” case was the first modern case to which that label was appended: a sick child whose physical signs and symptoms were collectively unrecognized as an entity at local and regional medical facilities.
As the child’s illness persisted without recognition or resolution, the unaffected parent sought similar illness descriptions from historic medical references, eventually settling on “The Morgellons”, a label given to childhood cases described in France in 1674 by Sir Thomas Browne [1]. His description was limited to its dermal components: hair-like extrusions and sensations of movement. Ettmuller, a physician, produced the only known drawings of The Morgellons in 1682 and considered these dermal “filaments” a parasitic infestation [1].
Numerous isolated descriptions of similar phenomena were found by Kellett from 1544 through 1884 that began as a pediatric-only illness identified by posterior torso “bristles” but also included extreme agitation, seizures, “wasting” and death (characteristics not seen in the 21st century).
By the early 1600s, the illness was thought to be caused by the parasite Dracunculus (later called Dracontia, then pilaris affectio) and “cured” by filament (agent) removal from the skin.
The worm theory was considered debunked by 1715 when the esteemed microscopist, Leuvenhoeck, pronounced the bristles “inanimate”.
From this point forward to 1884, however, the illness shifted toward comedones, facial regions and to adults. And Demodex folliculorum was considered a frequent occupant of comedones, again supporting a “parasite” concept.
The name “Morgellons disease” was thus created in 2002, as a practical “place holder” because of its dermal similarity to The Morgellons described by Browne in particular (Browne’s The Morgellons label evolved further over three centuries into the words, “Masquelon”, then Masclous dicti and Les Crinons, shifting to Comedones after 1800).
The new label also provided an alternative to differentiate a clearly, non-delusional child from many decades of clinical use of the label Delusions of Parasitosis [2-10].
This first set of actions would eventually raise one of the major philosophical questions of modern medicine:
Does the practice of “evidence-based medicine” come from peer-reviewed literature, or from the actual patient? Information from what was later to become the Morgellons Research Foundation (MRF) website initially provided a broader look at the believed Morgellons disease symptoms by allowing patient registrants to record their symptoms and signs.
Clearly, most of the 21st century patients did not fit the descriptions from 1500-1800.
The MRF website provided a glimpse of the current global prevalence of those considering they are affected by this phenomenon. On January 2009, there are 13,000+ family registrants from all U.S. states and from 15 other countries.
[http://www.morgellons.com/] Given the growing use of the label Morgellons disease, characterizing the illness using verifiable clinical data seemed to be the imperative first step for clinicians, particularly to separate these patients from those two to five centuries before presentation.
Until 2007, Morgellons and DP cases were often seen in isolation, interfering with credible Case Definitions. Assembling even this small number of candidate patients into a series with verified data points, should at least improved diagnosis consistency.
Case Series Data Collection
The study protocol was simply a collection of clinical data obtained from new Morgellons disease patients at one clinic in a two-hour session between September 2006 and July 2007.
Patients were not solicited but came self-diagnosed.
Practice size-limited the original patient number to 30 adults.
The final number was 25 after filtering for consistency and data errors.
Each clinical session included a detailed history, review of systems and a physical examination emphasizing dermal, neurological, endocrine and psychiatric systems.
Expanded laboratory tests included a blood count (CBC), a metabolic panel (CMP) and others to evaluate major organ system function to characterize immune status, detect inflammation and search for an initial and limited number of possible infectious pathogens suggested by several veterinarians [personal communication].
Subjective screening criteria derived from earlier clinical encounters were first applied to improve patient selection consistency (Table 1).
The screened data were then entered into 492 fields on each patient, 407 fields of which contained useful data points. This provided 10,175 total parameters for analysis.
Case Series Initial Data Summary
The first-level summary of raw data is in the Appendix, Tables A, B and C. Categories devoid of entries had no significant findings.
Table 1.
Initial Patient Screening Criteria
1. Patient convinced of chronic parasite infestation.
2. Primary patient illness focus must be either on dermal sensations or on “never-before-seen” material thought to be extruded from his or her body, even if this was not the most debilitating symptom or sign. The material must have been described as “fibers”, “fiber-like”, or “filaments”.
3. Chronic pruritis (itching) must have been present for at least six months.
4. The patient must have at least two chronic, unhealed skin lesions recorded by a clinician, regardless of whether excoriation was suspected.
5. Prior diagnosis of Delusions of Parasitosis or bipolar illness would not exclude the patient as a candidate.
6. Patients experiencing delusional states common in withdrawal from drugs such as opiates and patients in organic brain states, were excluded.
7. The illness must have had a life-altering effect on the patient.
8. The patient must be an adult and had experiencing symptoms for more than six months.
9. A healthy pre-morbid period in the patient’s life was acceptable.
A. Demographic Information
Male and female rates were the same for those who believed they had Morgellons disease.
Age spread, geographic spread and gender neutrality were large. These data suggest Caucasian bias that did not appear in data from other countries [11].
B. Illness History
Rural residence or recent rural travel was common.
Emotional stress was not relevant, nor was season of onset. Physical stress was a common precursor.
Full onset typically required one to several months from first symptom.
C. Past Medical History
Prior psychiatric diagnoses in more than half. The most common diagnosis was bi-polar illness.
D. Social History
Significantly reduced exercise capacity was highly prevalent although non-specific as an illness marker. Recent exposure to unhygienic settings (Third world countries, sewage systems and land fills) correlated strongly with illness onset.
E. Systems Information from physical examination and History of Present Illness General:
Weight gain after illness onset averaged to 33 pounds.
High levels of fatigue and reduced exercise capacity were eventually present in 80% of patients.
Recurrent fever by thermometry was noted by 50% of patients.
Dermatological:
A typical phrase used by most patients was similar to: Extruding and moving skin “parasites” (filaments, “fibers”, “spheres”) generating uncomfortable lesions. Patients stated “filament” appearance was intermittent and consistent with identifying in less than one in three patients on examination. The sensation of movement, however, was denied by up to 50% of those experiencing filament extrusions.
About 70% had regular appearance of painful shallow skin ulcers, but most could easily separate excoriations (scratching) they had generated from spontaneous ulcers.
Numerous micro-angiomas (0.5 to 3.0 mm in diameter) were found by examination on 72% of patients. This phenomenon is a known hallmark of Bartonellosis, although our patients had completely negative Bartonella serology [12,13].
Many patients noted that angiomata appearance directly paralleled illness onset and progression.
Central Nervous System:
This group experienced a high rate of headaches, visual aberrancies, tinnitus, and short-term memory deficits.
Emotional lability was present in more than half, typically manifest intermittently.
Cardiovascular:
Patients often reported orthostatic intolerance and frequent arrhythmias (type undetermined) regardless of age.
Rhythm and mild cardiac auscultation abnormalities (valvular) were commonly found on vital sign determination.
Pulse was high (>72) in virtually all.
Psychiatric:
Strikingly, most patients in this study (23 out of 25) had prior psychiatric diagnoses (most determined by specialists) as follows:
11 out of 25 bipolar disease;
7 out of 25 Adult ADD;
4 out of 25 Obsessive Compulsive Disorder (OCD);
and 1 out of 25 Schizophrenia.
Although overlap occurred in 5 cases, only the primary psychiatric diagnosis was tabulated [14].
In each case, medical records indicated that the dermal symptoms and signs preceded or occurred simultaneously with the onset of emotional signs, with an emotionally “normal” time in each patient’s life prior to Morgellons disease.
Endocrine:
Eight patients (32%) had prior diagnoses of Hashimoto’s Thyroiditis. [The U.S. adult prevalence rate of Hashimoto’s Thyroiditis is 0.56%. The rate is based on 1996 statistics: 1,490,371 adults with Hashimoto’s Thyroiditis per U.S. population of 264,162,000. (Reference: Rose and Mackay, 1998, The Autoimmune Diseases, Third Edition)] Half had past evidence of Hypercalcemia (intermittent), of which 3 had definite parathyroid adenomas, later surgically excised with incomplete improvement.
Fasting insulin levels were elevated in 100% who were tested for it (6 out of 25), as were CRH levels (also 6 out of 25 tested).
Other:
Recurrent cough and dyspnea were common as GI and urinary symptoms, but none had a recent medically determined etiology.
F. Vital Signs
The most Consistent markers in this group were low core temperature and high resting heart rate, affecting all 25 patients.
G. Laboratory Data
CBC aberrancies were common but often intermittent. They included abnormal variable RBC indices, occasional low-grade anemia, low white cell count, and high monocyte count.
Other abnormalities included low Natural Killer cell (CD56 + CD16) number and percentage, high or high-normal insulin level (in all tested) and intermittent elevation of serum calcium, globulin level, and A/G ratio. Sedimentation rate was mid-range “normal” or lower with only one ANA positive. Antidouble stranded DNA antibody level was negative in all tested. Occasionally IgG subclasses 1 and 3 were low. Commonly elevated markers supporting chronic inflammation included C-reactive protein and TNF-alpha.
Other occasionally abnormal laboratory parameters present in chronic inflammation or infection included IFN-gamma, Homocysteine and serum Leptin. Most patients showed serologic evidence of infection (antibodies) with one or more unexpected potentially pathogenic microorganisms despite testing for only a few species.
Data Summary
Following is the concluding summary of collected data from all patients, including pathological mechanisms suggested by the pattern(s) of these data anomalies.
Morgellons disease was often preceded by exposure to unhygienic conditions and appears first as skin abnormalities and discomfort.
Illness onset appears with moderately rapid transition (weeks) from healthy to unhealthy, including “emotional discomfort”.
These gravid Morgellons females had an extraordinarily high miscarriage rate.
The most common physical abnormalities found in this series include:
1) Numerous “senile angiomas” on the trunk, head and limbs of many;
2) Recurrent fever;
3) Awareness of itching, crawling, stinging or biting. When present, patients describe a circadian tempo to the symptoms; some occurring solely at night. Itching of unbroken skin specifically appeared to precede all other skin symptoms;
4) Unidentified objects (called “filaments” or “granules”) “extrude” uncomfortably from unbroken skin or skin lesions;
5) Painful ulcer-like concave, circular skin lesions with distinct border;
6) Excoriations adjacentto but separate from ulcerations were common;
7) Dermal symptoms were the central focus of discomfort for most patients.
8) Multiple organ system symptoms often appeared within the first six months of illness onset.
The most common systems affected were the central and peripheral nervous systems, autonomic nervous system then endocrine, cardiovascular, and pulmonary systems.
All blood pressures were low and all resting pulses were high.
Routine laboratory tests were often inconsistent and varied both positively and negatively, but within range more frequently than out of range.
Common abnormalities were NK cell numbers and percentages (low), and fasting insulin levels (very high).
Occasionally abnormal were RBC indices, hematocrit, WBC count (low), monocyte count (high), serum calcium (high), globulin level (high), and A/G ratio (high). Contradictory autoimmunity data was frequently noted.
Some expected inflammation tests such as sedimentation rate and Anti-double stranded DNA antibody tests were negative, while C-reactive protein and TNF-alpha were routinely high. IFN-gamma, Homocysteine and Leptin were also elevated.
There was a high prevalence of transient “autoimmune” diseases such as Hashimoto’s Thyroiditis, hyperparathyroidism and adrenal-cortical hypofunction.
Following is a second level summary of the Morgellons data (Table 2).
This is intended as a simplistic tool for clinical use. Conclusion Proposed Characterization of Morgellons Disease
The authors conclude that Morgellons disease is a multi-systemic illness that has been presumed as a delusional phenomenon for decades as its most obvious and disconcerting manifestations resembled actual (but “unverified”) parasite infestation as well as various psychopathologies.
However, using recent technology and even a modicum of consistently obtained physical data supports that Morgellons manifest as a skin phenomenon, an immune deficiency state and a chronic inflammatory process.
Since infectious agents can initiate and maintain chronic diseases, the behavioral and other CNS manifestations here are more likely effect than cause [18]. We suggest that the Morgellons label be considered to displace any label suggesting delusion as the primary cause of this phenomenon.
The term “Morgellons disease” came into being in the 21st century in an attempt to identify an illness for which no name existed. The entry of this new label into a phenomenon of extreme controversy may at first appear to further that controversy. Typical of the evolution of medical nomenclature, however, the problem may always have been with semantics; in particular the use of assumptions unsupported from failure to investigate the total physical patient [19].
The “index” case that failed to fit similar earlier diagnostic labels was seen as “different” principally because its observer looked beyond the presumed signs and symptoms of the truncated “look-alike” phenomenon labeled “Delusions of Parasitosis”.
Trabert’s review of 1,300+ cases makes clear that only a fraction of the total signs of that presumed illness were used to create the DP diagnostic framework [14,20].
Our attempt to gather as much physical evidence on Morgellons patients as possible was based on the extremely large number of abnormal physical signs among those we evaluated earlier. We gathered as many clinical parameters as possible (within the fiscal constraints of “today’s” medicine) in order to see whether the abnormalities among them were consistent and if so, whether their pattern was explanatory. The unfolding mechanism strongly suggests a chronic infectious process.
Table 2.
Primary Abnormal Findings
1. The large age spread, geographic spread and gender neutrality among patients suggest broad human susceptibility to the illness. 2. Rural abode or exposure to unhygienic conditions (third world travel or simply soil exposure) may be risk factors.
3. Onset rate is moderately rapid, without recognizable prodrome, commonly preceded by a healthy state.
4. Once ill, exercise capacity is significantly reduced.
5. The illness is common among family members and close associates, both related genetically and unrelated (such as spouses).
6. Most patients experience weight gain after disease onset.
7. Micro-angiomas appear rapidly on skin after illness onset in most.
8. Fever is recurrent in at least half of those affected.
9. The first illness sign may be the sudden appearance of persistent itching. Ulcerative lesions follow in some cases.
10. Once dermal symptoms begin, patients experience extrusion of unfamiliar material described variously as filamentous, “fuzz balls”, black or white “flecks” or “rice grains”.
11. Numerous CNS effects occur, that includes bizarre cranial nerve phenomena, anxiety and emotional sequelae. The former tend to be transient.
12. Numerous Peripheral Nervous System findings appear after illness onset. Unlike CNS effects, these are serious, permanent and progressive, and include sensory and motor nerves.
13. All Morgellons have elevated heart rate (>72 BPM) and low body temperature by oral thermometry (<97.5 degrees F).
14. Orthostatic intolerance is intermittent but common in most.
15. Most have some formally diagnosable emotional illness that begins with or becomes apparent after Morgellons disease onset. 16. Endocrine abnormality number and type is higher than background. Most common are Diabetes Type II, Hashimoto’s Thyroiditis, hyperparathyroidism and adrenal hypofunction.
17. Most have elevated fasting insulin levels accompanied by elevated TNF-alpha (insulin receptor blocker) [15,16].
18. Common clinical laboratory abnormalities include: a. RBCs have abnormal morphology. On manual examination, RBCs were non-discoid, varied in color and size. b. Natural Killer Cell (CD 56 + CD 16) number and function are very low in most. c. A/G ratio and globulin level are frequently elevated. d. Sedimentation Rate and ANA are extremely low despite other common autoimmune-like conditions. e. Elevated cytokines include: TNF-alpha, IFN-gamma, IL-6, C Reactive Protein, Homocysteine and serum Leptin.
19. Despite no fact-based Case Definition of Delusions of Parasitosis (DP), each of our 25 patients could have been given such an illness label as well. As pointed out by Trabert in a meta-analysis of 1,223 DP cases and others, most DP data were taken from isolated cases. Psychiatric-skewed labels were common such as “psychocutaneous disease”, “acarophobia” and “monosymptomatic hypochondriasis” with no serious search for consistent physical abnormalities or microscopic parasitic agents [17].
The specific agent candidates will not be addressed further until evidence of their presence is available and their presence can explain the signs and symptoms we now find in all Morgellons patients. There remains considerable work to do in collecting more data from these patients to create a credible Morgellons disease Case Definition. We submit that the same holds true for Delusions of Parasitosis patients [3]. Much of that work may be now under way by the U.S. Centers for Disease Control and Prevention (CDC) through contract with Kaiser Permanente’s Northern California Division of Research. [http://www.cdc.gov/unexplaineddermopathy/]
Meanwhile, the consistent abnormal findings in the data above may be used to improve clinical diagnosis and possibly initial treatment in current patients. Perhaps of considerable importance to a journal dedicated to Case Reports is what the juxtaposition of Morgellons disease and Delusions of Parasitosis suggests.
As noted by Trabert in his meta-analysis, creating a Case definition primarily from isolated cases allows uncontrolled use of assumptions that vary considerably in order to keep an unresolved conclusion constant. Where many cases are used, consistency of similar data forces a far more valid and consistent conclusion [14,20].
Until the machinery of science is in full gear and provides understanding of this phenomenon, simply “paying attention”, maintaining skepticism, practicing a simple physical exam and using commercial laboratories judiciously must suffice.
Once the breadth and severity of what we are encountering is understood, the resources and motivation for its solution should come. When sufficient, we anticipate the framework of several medical specialties may be modified.
Consent
Written informed consent statements were obtained from the patients for publication of this case report. The copies of the written consent are available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they do not have any competing interests.
Authors’ contributions
WH crafted the submission in its final form, including the cover letter, abstract, title page and references, drafted the summary and conclusions, and submitted the package to JMCR. RB assisted with the completion of the entire submission and was responsible for all sections addressing psychiatric elements of the illness, particularly references to Delusions of Parasitosis.
More will follow in paper #2. DM was responsible for all references to known parasite species and all conclusion statements considering parasitosis. AW analyzed all micrographs from several fluid and tissue sources for pathogen identification and contributed data from his own pool of anonymous UK patients for context. RR obtained medical histories and systems review from all series patients and applied her FBI forensics scrutiny training to complete a second physical examination on each patient. ML is the creator of the current Morgellons label and concept, and until characterized systematically, its sole “authority”. She created the Morgellons registration website, and modified this submission for consistency with the 1300+ site registrants.
All authors read and approved the final manuscript.
Acknowledgements None.
References
1. Kellett C: Sir Thomas Browne and the Disease called the Morgellons. Annals of Medical History 1935, 7:467-479.
2. Robles DT et al.: Delusional disorders in dermatology: a brief review. Dermatol Online J 2008, 14:2.
3. Berrios GE: Delusional parasitosis and physical disease. Compr Psychiatry 1985, 26:395-403.
4. Szepietowski JC et al.: Delusional parasitosis in dermatological practice. J Eur Acad Dermatol Venereol 2007, 21:462-465.
5. Nicolato R et al.: Delusional parasitosis or Ekbom syndrome: a case series. Gen Hosp Psychiatry 2006, 28:85-87.
6. Aw DC, Thong JY, Chan HL: Delusional parasitosis: case series of 8 patients and review of the literature.
Ann Acad Med Singapore 2004, 33:89-94.
7. Koo J, Gambla C: Delusions of parasitosis and other forms of monosymptomatic hypochondriacal psychosis.
General discussion and case illustrations. Dermatol Clin 1996, 14:429-438.
8. Johnson GC, Anton RF: Delusions of parasitosis: differential diagnosis and treatment. South Med J 1985, 78:914-918.
9. Amato Neto et al.: Ekbom syndrome (delusory parasitosis): ponderations on two cases. Rev Inst Med Trop Sao Paulo 2007, 49:395-396.
10. Bouree P, Benattar B, Perivier S: Ekbom syndrome or delusional parasitosis. Rev Prat 2007, 57:585-589.
11. Savely VR, Leitao MM, Stricker RB: The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol 2006, 7:1-5.
12. Podsiadly E, Chmielewski T, Tylewska-Wierzbanowska S: Bartonella henselae and Borrelia burgdorferi infections of the central nervous system. Ann N Y Acad Sci 2003, 990:404-406.
13. Brouqui P et al.: Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine (Baltimore) 2005, 84:61-68.
14. Aaron LA, Buchwald D: A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001, 134:868-881.
15. Holden RJ, Pakula IS, Mooney PA: Tumor necrosis factor-alpha: a continuum of liability between insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus and carcinoma (review). Med Hypotheses 1999, 52:319-323.
16. Nishimura M et al.: Role of tumor necrosis factor-alpha in insulin sensitivity and effect of low protein diet on the TNFalpha response in patients with diabetic renal failure. Nippon Jinzo Gakkai Shi 1997, 39:740-745.
17. Trabert W: Epidemiology of delusional ectoparasitic infestation. Nervenarzt 1991, 62:165-169.
18. Ewald PW: The evolution of virulence. Sci Am 1993, 268:86-93.
19. Ruchlis H: Clear Thinking: a practical introduction. 1st edition. 1990, Buffalo, NY: Promethius Books, 1990:271.
20. Trabert W: 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology 1995, 28:238-246.
APPENDIX TABLES OF RESULTS NOTE:
These tables are a first level summary of the complete basic data set except for physical examination findings that are summarized above. Although subjectivity has been introduced, we have attempted to keep it separate as footnotes.
Table A.
Basic Background Data
Demographics Countries of origin: U.S., U.K., Canada, Japan
Male: Female ratio approximates 50:50
Age range: 10-75 years
24/25 were Caucasian
Urban: Rural ratio = 50:50
Past medical history
11 out of 25 were diagnosed with bipolar disease
7 out of 25 were diagnosed with adult ADD
1 out of 25 was diagnosed with schizophrenia
Common childhood illnesses were absent regardless of vaccination status
19 out of 25 had been given general anesthesia at least once
9 out of 25 had prior orthopedic surgery
6 out of 25 had other prior surgery for chronic inflammatory diseases (appendix, gallbladder)
Illness History
Time from initiation to full onset: several months
Exposure to unhygienic conditions occurred several days to several weeks prior to illness onset
Patients claimed relative healthiness prior to Morgellons (as initially described) onset;
No consistent recognized prodrome heralded onset of the illness
Allergy symptoms were rare
Activating stressor event was present in 1 out of 3 of cases
Social History
50% were married/ 50% single
28% were smokers
None routinely exercised
Highest relevancy results.
Findings that may help to isolate, identify, and define Morgellons disease.
Other data.
1 This sample was not adequate to determine whether race is a factor. The high percentage of Caucasian patients was likely related to clinic location. Earlier and subsequent data of larger numbers from more heterogeneous regions suggest that race is likely not a risk factor.
2 The urban to rural ratio may be significant. Although only 20% of people live in rural areas, 50% of the patient samples were in rural regions near the onset of their illness, lending support to the hypothesis that exposure to unhygienic conditions or animals may be a risk factor.
Table B.
Review of Systems
Dermatologic
24% of patients had dark filaments visibly protruding from the skin
17 out of 25 had frequent skin eruptions/rashes
Just over 50% experienced no movement sensations
Only 50% itched regularly 72% has angiomata appear
Central Nervous System
Over half had frequent headaches
Over half had unstable visual acuity including double vision (10 out of 25)
10 out of 25 reported visual “flashes” (not characterized) in either daylight or dark
Emotional liability in half of patients
1/3 reported frequent dizziness
1/3 reported permanent hearing loss (degree not determined)
40% reported tinnitus
20 out of 25 reported frequent occurrence of forgetfulness
12 out of 25 had persistent regional skin numbness
6 out of 25 had persistent tremors
Metabolic Average weight gain after onset:
33 lbs
20 out of 25 experienced regular high levels of fatigue
25% were intolerant to cold and
25% intolerant to heat (some overlap, but degree undetermined)
10 out of 25 reported sweating inappropriately (either under- or over-sweating)
Immune
12 out of 25 had cyclical fever
18 out of 25 reported numerous angiomas verified by examination
10 out of 25 reported many “colds” annually
8 out of 25 reported “chronic sinusitis”
4 out of 25 reported frequent sore throats over a period of years
2 out of 25 reported frequent toothaches
Endocrine
8 out of 25 had prior diagnosis of Hashimoto’s Thyroiditis
50% had hypercalcemia,
3 of whom had parathyroid adenomas
Fasting insulin levels were elevated in all tested (6 out of 25)
Corticotrophin Releasing Hormone (CRH) levels were elevated in all tested
Musculo-skeletal
15 out of 25 reported frequent neck pain
10 out of 25 had persistent limited neck movement
22 out of 25 had chronic musculoskeletal pain,
without arthritis Pain was constant and incapacitating in 1/3 of patients
Gastro-intestinal
50% had constipation or diarrhea, or both (overlap was common)
25% had chronic nausea
Pulmonary Persistent cough (non-productive, non-wheezing) in 9 out of 25
Persistent dyspnea in 7 out of 25
Emotional
Half reported “anxiety”
Half reported frequent mood swings
Half reported frequent depression
Other
Urine stream control problems in 6 out of 25
1 Less than half of the 25 patients studied reported the presence of filaments, and as a result, the authors did not search for filaments on all patients.
2 Average weight gain in male and female patients combined. Time period for weight gain undetermined.
Table C.
Vital Signs and Laboratory Data Lab Parameters Laboratory Values
Vital Signs
Average temp 97.51° F
Average pulse 85.32 BPM Laboratory Data
Unidentified lymphatic filamentous clusters were found in 4 patients
50% were positive for Borrelia burgdorferi sensu lato either by CDC Western Blot (WB) criteria, EUCALB WB (IgM P31 and 34 added), or IFA
4 patients were seropositive for Babesia microti 92% were seropositive for Chlamydia pneumonia (Chp)
1 Suggests Borrelia may contribute to some cases of Morgellons disease.
2 Suggests Babesia is uncommon in most Morgellons disease cases.
3 Suggests Chlamydia pneumonia (Chp) is one candidate for the initial etiology of Morgellons disease via generation of an immunodeficiency state.
4 Lab tests were drawn at 6,100 feet MSL (above mean sea level).
5 RBC characteristics were adjusted for elevation. All indices should be “normal.”
6 Lower than regional mean of 66%.
7 Slightly higher than regional mean of 28%.
8 Much higher than regional mean of 3.0%.
9 Although a broad “normal” range exists for NK number, our clinical experience supports that consistently healthy individuals who rarely experience viral syndromes have NK # > 200. Our patients’ range is not infrequently below 10. This suggests one component of an immune deficiency state.
10 Well above regional mean of 84.
11 Well above regional mean of 0.6.
12 Although clearly “within range” at 6,100 ft. MSL, the mean of more than 100 similar patients tested near sea level (2001-2004) was 18 (21-28 anticipated). As low CO2 level translates to low serum bicarbonate level, this suggests required buffering to lower acidity. Although there are several causes of elevated serum acidity, many of these patients underwent overnight studies for sleep apnea that revealed consistent low breathing rate raising PCO2. No such formal test was included in testing these patients although we believe such testing would be highly revealing.
13 Despite this average being satisfactory, 7 of the 25 patients tested were between 10.0 and 10.6. Three of 25 patients were found to have parathyroid adenomas.
14 Greater than 5.0 in 9 of 25.
15 The converse of immune deficiency, however, suggests that a possible chronic infectious state may have been operant that could intermittently activate humoral immunity.
16 Commonly above 2.0. As globulin is elevated and albumin is clearly not low, the ratio elevation is driven somehow by the albumin. This is presently a paradox.
17 Average is high normal and well above the regional mean of 68. Suggests skeletal involvement in the disease, although liver involvement is possible. Most Morgellons patients have shown evidence of osteopenia or osteoporosis when randomly tested despite age. Bone density was not determined in these 25 patients. We believe doing so would be valuable.
18 Argues against an autoimmune role, particularly as 5 out of 25 were 0, and all were less than 20.
19 Despite this normal finding, Hashimoto’s Thyroiditis is common in larger similar groups of Morgellons. Further evaluation of the HP axis and the endocrine system in general should be included in any future studies.
20 Currently used to assess cardiac risk in three stages. In this context, we are assessing chronic inflammation. This average supports the presence of a persistent inflammatory process that parallels physical evidence of vasculitis.
21 Five >150, suggests cytokine activation and possible inflammatory effect.
22 High-normal. 11 out of 25 >3.6, suggests cytokine activation and possible inflammatory effect.
23 Definite elevation in 92%. Most commonly corresponds to insulin receptor blockade and to chronic inflammatory effects.
24 Elevation of Transforming Growth Factor beta (TGF-beta) can occur when pathological events diminish its protective growth modulating effects on various tissues. This test result parallels evidence of excessive growth processes observed in these patients such as numerous angiomata, skin tags, nevi, and regions of increased epidermis density.
25 Consistently elevated in most male and female study patients. Parallels the gain in body fat and angiogenesis experienced by most following illness onset.
A Chemical and Light Microscopic Study
Marianne J. Middelveen 1, Elizabeth H. Rasmussen 2, Douglas G. Kahn 3 and Raphael B. Stricker
1* 1 International Lyme and Associated Diseases Society, Bethesda, MD 2 College of Health Sciences, University of Wyoming, Laramie, WY 3 Department of Pathology, Olive View - UCLA Medical Center, Sylmar, California
http://www.omicsonline.org/2155-9554/2155-9554-3-140.pdf
Abstract
Morgellons disease is an emerging multisystem illness characterized by unexplained dermopathy and unusual skin associated filament production. Despite evidence demonstrating that an infectious process is involved and that lesions are not self-inflicted, many medical practitioners continue to claim that this illness is delusional. We present relevant clinical observations combined with chemical and light microscopic studies of material collected from three patients with Morgellons disease. Our study demonstrates that Morgellons disease is not delusional and that skin lesions with unusual fibers are not self-inflicted or psychogenic. We provide chemical, light microscopic and immunohistological evidence that filaments associated with this condition originate from human epithelial cells, supporting the hypothesis that the fibers are composed of keratin and are products of keratinocytes.
Keywords:
Morgellons disease; Digital dermatitis; Lyme disease;
Borrelia burgdorferi; Spirochetes; Keratin
Introduction
Morgellons disease (MD) is an emerging dermatological disorder and multisystem illness. The disease is characterized by unexplained dermopathy associated with formation of unusual filaments found both subcutaneously and emerging from spontaneously appearing, slow-healing skin lesions [1]. Filaments associated with MD appear beneath unbroken skin [1,2], thus demonstrating that they are not self-implanted.
Filaments have been observed protruding from and attached to a matrix of epithelial cells [3]. This finding demonstrates that the filaments are of human cellular origin and are not textile fibers.
These filaments have not been matched with known textile fibers, and dye-extracting solvents have failed to release coloration; the fibers are also very strong and heat resistant [4,5]. MD filaments are physically and chemically consistent with keratin, a biofiber produced in the epithelium by keratinocytes. A recent report from the Centers for Disease Control and Prevention (CDC) confirmed that these filaments have a protein composition that is consistent with keratin [6].
Lyme disease-like symptoms in MD such as neurological disorders and joint pain are evidence of systemic involvement [1,2,7]. Objective clinical evidence of disease has been demonstrated by its association with peripheral neuropathy, delayed capillary refill, decreased body temperature, tachycardia, elevated pro-inflammatory markers, cytokine release, selective immune deficiency and elevated insulin levels, suggesting that an infectious process is involved [8,9]. Patients may demonstrate abnormal laboratory findings indicative of low-grade anemia, endocrine dysfunction, immune dysfunction and inflammation [8,10]. Patients with MD are predominantly sero-reactive to Borrelia
burgdorferi (Bb) antigens, suggesting a likelihood of Lyme borreliosis or related spirochetal infection [1,10]. Patients also demonstrate a higher than expected percentage of positive laboratory findings for other tick-borne diseases, suggesting the possible involvement of coinfecting pathogens [10].
The observation of unusual filaments forming in lesions is not unique to humans afflicted with MD. Similarities between MD and bovine digital dermatitis (BDD) have been described [3]. BDD is an emerging disease afflicting cattle and is characteristically associated with unusual filament formation in skin above the hooves [11]. Latestage proliferative lesions demonstrate elongation of keratinocytes, hyperkeratosis, and proliferation of long keratin filaments [12-14]. Consistent detection of spirochetes associated with lesions is evidence of spirochetal etiologic involvement [15-20]. Experimental induction of lesions with tissue homogenates [21] and pure cultured treponemes [22] supports a role for spirochetes as primary etiologic agents.
Like BDD, MD is associated with apparent spirochetal infection and unusual filament production [3]. A comparison between BDD and MD suggests that the unusual fibers seen in MD patients may result from hyperkeratosis and filament production as described in BDD. It appears that MD fibers are likewise composed of keratin produced by
keratinocytes, a phenomenon that has been demonstrated in BDD [3]. The following three case studies provide further evidence supporting this hypothesis.
Materials and Methods
Human and bovine samples
Three patients meeting the clinical criteria for Morgellons disease collected calluses, scabs, filaments, and other dermatological debris and submitted the material for microscopic examination. The collected samples were examined by bright-field microscopy at 100x magnification. Specimens were illuminated either superior or posterior to the specimen. Some specimens were also illuminated with ultraviolet (UV) light.
Biopsies from cattle with BDD were kindly provided by Dr. Dorte Dopfer, Faculty of Veterinary Medicine, University of Wisconsin, Madison, WI. Biopsy material from proliferative late stage BDD was examined for comparison to MD samples with 8x magnification under a dissecting microscope. This material was also tested for fluorescence under UV light.
For the chemical experiments, samples of normal hair, filaments from Cases 1 and 2 and BDD fibers were studied for reactivity to three caustic agents: sodium hypochlorite 12%, sodium hydroxide 10%, and potassium hydroxide 10%. Each sample was suspended in 150 µl of the chemical solution for up to two hours, and serial light microscopy was
performed at 0, 1, 10, 30, 60 and 120 minutes. Dissolution of fibers was assessed by fraying, loss of shape and/or disintegration at each timepoint.
For the immunohistological experiments, filament samples from Cases 1 and 2 were stained for keratin using monoclonal antibodies. Briefly, formalin-fixed paraffin-embedded filaments were incubated with monoclonal antibodies AE1/AE3 (Dako North America Inc, Carpinteria, CA) and AE5/AE6 (Cell Marque Corporation, Rocklin, CA) directed against cytokeratins 1/3 and 5/6, respectively, using the Envision® + Dual-Link System-HRP (Dako) according to the manufacturer’s instructions. The samples were stained using a horseradish peroxidase label, and the brown staining of keratin was visualized under light microscopy
Clinical Observations
Case 1
The patient is a 72-year-old grandmother and former fashion model who developed painful lesions on her hands while working in her garden in San Antonio, Texas, in 1994.
The lesions were punctate with ragged edges and healed slowly, leaving visible scarring. Fibers were observed in the lesions and under intact skin on her hands using a 60x handheld microscope. Topical steroids had no effect. The patient also noted the onset of fatigue, joint pain and muscle aches, and systemic steroid treatment exacerbated these symptoms without any improvement in the skin lesions. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation was entirely normal. Biopsy of a lesion demonstrated hyperkeratosis and parakeratosis with no visible organisms or evidence of vasculitis. However “textile fibers” were noted in the dermal layer of the biopsy specimen.
In 2001, after numerous visits to dermatologists and other medical specialists and treatment with topical emollients and antiinflammatory medications, the patient had persistent skin lesions on her hands, fatigue and musculoskeletal pain. Despite the use of gloves to avoid scratching, her lesions persisted and she was unable to work in her garden or hold her grandchildren due to pain in her hands and joints. She recalled numerous tickbites but never saw an erythema migrans (EM) rash, and she was found to have positive testing for B. burgdorferi, Babesia microti and Bartonella henselae. She was treated with antimicrobial medications and her fatigue and musculoskeletal pain improved significantly. However her skin lesions persisted. She received anti-parasitic medication, and the lesions improved to the point that she could once again do gardening. The lesions persist but are “manageable” (Figure 1A).
Case 2
The patient is a 49-year-old registered nurse who had numerous tickbites while hiking, camping and horseback riding in Missouri, Texas and Northern California over more than a decade. She never saw an EM rash. In 1997 while living in San Francisco she eveloped painful lesions on her face, trunk and extremities. The lesions were punctate with ragged edges. Some lesions healed slowly, leaving visible scarring, while others did not heal at all, and fibers that were resistant to extraction were observed within several lesions. Fibers were also observed under intact skin using a 60x handheld microscope.
Topical steroids had no effect. Biopsy of a lesion on her leg revealed hyperkeratosis and parakeratosis without evidence of infection or vasculitis. However, “textile fibers” were noted in the dermal layer of the biopsy specimen. She also developed fatigue and musculoskeletal pain, and systemic steroid treatment exacerbated these symptoms without any improvement in the skin lesions. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation was completely normal.
Because of persistent fatigue, musculoskeletal pain and her history of tick exposure, the patient was evaluated for Lyme disease in 2004 and had positive testing for B. burgdorferi and Ehrlichia chafeensis. Antibiotic therapy led to improvement in the fatigue and musculoskeletal pain, but the skin lesions persisted. She received antiparasitic medication and her skin lesions improved somewhat, but new lesions appeared and healing lesions caused painful scarring. She has received intermittent courses of antibiotics over the past six years, and her skin lesions continue to wax and wane.
Case 3
The patient is a 47-year-old business manager who was in excellent health until he developed a “bullseye” rash, fever, chills, severe headache, musculoskeletal pain and malaise after hiking in the woods near Atlanta, Georgia, in 1995. He had pulled ticks off his dog, which also became ill at the same time. He was diagnosed with fibromyalgia and treated with pain medications, but by 2000 he had become progressively disabled by muscle pain and fatigue.
In 2002 he developed crawling sensations on his head, face, groin and other body areas where there was hair. The sensations were accompanied by painful skin lesions. He was diagnosed with folliculitis and put on a topical antibiotic, which made his skin symptoms worse. He began to notice painful fibers coming out of the skin on his face, head and other hirsute areas, and he could not sleep because the fibers were so painful.
He extracted fibers from his facial lesions, but new ones appeared. He was diagnosed with trichotillomania and delusional parasitosis.
He went to several dermatologists and was treated with topical lindane and oral cephalexin without benefit. Treatment with oral ketoconazole and fluconazole provided marginal improvement in the crawling sensations and skin lesions. A scalp biopsy demonstrated increased numbers of catagen and telogen follicles with fragmented hair fibers and inner root sheath consistent with trichotillomania.
There were no visible organisms or evidence of vasculitis. Medical evaluation was negative for autoimmune or infectious diseases, and neuropsychiatric evaluation revealed reactive depression. He was treated with antidepressants without benefit.
Finally in 2005 a physician noted fibers under his skin using a 60x hand-held microscope.
Testing for Lyme disease was indeterminate in 2006, and treatment with doxycycline was given for one month without benefit. The patient continues to suffer from crawling sensations, skin lesions, musculoskeletal pain, disabling fatigue and depression. He is reluctant to see any more physicians about his skin condition (Figure 1C).
Results
MD Microscopic observations
Case 1: Microscopic examination revealed a wide range of filaments in various stages of formation ranging from early stages that demonstrated either single or clusters of hyaline, tentacle-like projections with tapered ends (tentacle diameter approximately 5 µm) to macroscopic masses or mats of tangled fibers (approximately 1 mm diameter). Floral-like formations of early-stage filaments were observed in some samples that were collected on different dates and years. These structures had tapered ends with bases originating at a central point and were found in groups anchored to a dried dermal matrix. The reverse side of some of these specimens revealed a layer of pavement epithelial cells. Epithelial matrices anchoring longer hyaline fibers were observed, suggesting that as the tentacle-like projections increase in length individual fibers may become tangled, or clumped. Various structures composed of clumps, strings, and nest-like balls of hyaline filaments were observed and some of these were glued together by clotted or dried exudate. This suggests that tangled filaments may eventually separate from the supporting epithelial matrix and form balls and other tangled structures.
Some samples revealed raised unidentified papules protruding from dried epithelial tissue that might be abnormal hair follicles. Long isolated colored filaments, filament fragments, balls, and clumps of fibers (red, blue, black and green) were also observed, but were not attached to or growing from epithelial tissue. Many of these colored filaments had bulb-like ends (50 µm diameter) that looked very much like those found in hair follicles.
Many fibers displayed iridescence under bright-field microscopy and were fluorescent under UV lighting. Hyaline or white fibers fluoresced brightly, as did blue fibers. Red and green fibers displayed striking iridescence but fluoresced with less intensity than the blue and white fibers. This suggests that melanin pigments may be associated with red and green filaments.
Early floral-shaped clusters were brightly fluorescent. Human hair was not fluorescent nor was normal skin. Color intensity and hue of the red and blue filaments was influenced by the color spectrum of the illuminating light. This property and the presence of iridescence suggests that a structural component is involved in the unusual colors
seen in Morgellons fibers.
Case 2: Microscopic examination of scab material revealed scab detritus imbedded with long filaments of various colors. Hyaline, red, blue, and light purple fibers were observed (10-40 µm diameter). One sample revealed fibers tangled around a hair and these fibers may have been associated with the hair follicle. Smaller, pale purple fibers (10 µm diameter) appeared to form a mesh around the follicle. Some samples revealed fibers that lay beneath or penetrated dermal tissue.
Case 3: Microscopic examination was performed with particular attention to hair follicles, as the patient had reported unusual filament formation associated with the follicles. Microscopy revealed abnormalities of the follicular bulbs and the hair associated with these follicles that indicated abnormal functioning of follicular keratinocytes.
Many follicles contained malformed bulbs with distorted shapes, and some follicles had two or more hairs branching from a single inner root sheath. Filaments stemming from the bulb end were found in some follicles and these appeared as rootlike growths. Transparent filaments were observed that stemmed from cells within the inner root sheath.
On some hairs red or blue colored filaments branched from the shaft. Many hairs were flattened or tape-like on cross-section rather than concentric. These hairs were similar in appearance to Morgellons filaments.
BDD Microscopic observations Biopsies from late proliferative stage BDD lesions were examined microscopically for comparison. Although the scale of filaments was much larger, the BDD filaments (roughly ten times larger) were similar in appearance compared to the specimens observed in Case 1. Filaments were macroscopic, opaque and dirty white in color, ranging in size from less than 0.5 mm in diameter to about 1 mm in diameter. In cross section filaments appeared to originate beneath the stratum corneum. Longer filaments were close to 1 mm in length. The BDD filaments demonstrated fluorescence under UV light.
Chemical Experiments
Samples of normal hair, colored filaments and dermal material from Cases 1 and 2, and BDD fibers were subjected to immersion in caustic agents. Duplicate experiments with each caustic agent were performed on each sample. Results of the experiments are shown in (Table 1) Normal hair and patient filaments began to fray after incubation for 1 minute, and the patient filaments had completely disintegrated after incubation for 120 minutes in 12% sodium hypochlorite. Normal hair was still visible at this timepoint. In contrast, patient filaments began to fray at 1 minute in 10% sodium hydroxide but were still visible after 120 minutes, similar to normal hair. The hair and patient filaments
were more resistant to 10% potassium hydroxide, with visible fraying beginning at 10 minutes and fibers still visible at 120 minutes. Although the larger BDD fibers appeared to be more resistant to the chemicals, fraying and shape change similar to the human samples was evident at 120 minutes with each caustic agent.
Keratin immunostaining
The results of keratin immunostaining experiments are shown. The MD filaments from Case 1 stained strongly with the “pankeratin” antibody AE1/AE3 directed against cytokeratin 1/3.
In contrast, the filaments stained weakly with the more restrictive antibody AE5/AE6 directed against cytokeratin 5/6. Staining with AE1/AE3 was seen over the length of the fiber, while staining with AE5/ AE6 was only detected in the outermost scale. Melanin pigmentation
was not seen in the fibers. No staining was detected with an irrelevant monoclonal antibody, and similar positive keratin staining with AE1/AE3 was detected in MD fibers from Case 2 (data not shown).
Discussion
Our three patients had features of MD that are commonly described in the medical literature, including insidious onset, dermatological signs and systemic symptoms, lack of response to immunosuppressive treatment and association with tickborne diseases [1-3].
Case 1 had skin lesions confined to the hands, while Cases 2 and 3 had disseminated skin lesions over the head, trunk and extremities. In addition, Case 3 had symptoms associated primarily with hair follicles, and a sensation of change in hair composition and texture is often reported by Morgellons patients [1,10]. These MD patterns have been recognized in prior studies [1,2] and we propose a classification of localized MD versus disseminated MD based on the distribution of the dermopathy. Although the reason for this dermopathy distribution is unknown, the location of skin lesions may be related to the cell of origin of the fibers seen in lesions or under the skin, as discussed below. Further study of the dermopathy distribution in MD is warranted.
The present study demonstrates Morgellons filaments that clearly originate from a layer of pavement epithelial cells visibly held together by desmosomes. The predominant cells found in pavement epithelial tissue are keratinocytes. We also noted MD fibers that clearly originate from the inner root sheaths of hair follicles, and keratinocytes are the predominant cell type in this tissue. Keratinocytes produce the biofiber keratin. A cross section of BDD filaments likewise demonstrates filament origin from cells beneath the stratum corneum, consistent with descriptions in the literature of growth from keratinocytes [14,19]. Thus MD filaments and BDD filaments appear to be similar in formation at the cellular level, both originating from keratinocytes in the stratum spinosum or stratum basale. MD differs from BDD, however, in that MD filaments appear to originate from follicular keratinocytes as well as epidermal keratinocytes. Both MD filaments and BDD filaments fluoresce in UV light. We have also shown for the first time that MD filaments contain keratin, and keratin staining was positive using a “pankeratin” monoclonal antibody but negative with a more restricted keratin ligand. This observation indicates that the fibers originate from specific tissues that require further characterization.
The observation that MD fibers are found beneath unbroken skin, may grow from an epidermal matrix and are associated with hair follicles suggests that they are not self-implanted textile fibers [1-3]. The filament formation described in MD is associated with a high likelihood of Bb infection [1,10]. BDD in cattle is associated with hyperkeratosis, keratin filament formation and spirochetal infection [12-20]. Hyperkeratosis and excessive keratin production associated with chronic inflammation has been demonstrated in humans with cholesteatoma [23,24], and alterations in keratinocyte expression of HLA markers and tissue enzymes have been reported in association with Bb infection [25,26]. These observations suggest that hyperkeratosis and keratin filament production associated with spirochetal infection is a plausible explanation for the clinical and microscopic findings in
MD.
Hyaline and colored filaments from the three case studies demonstrate iridescence and an appearance consistent with keratin. Red, blue, purple and black are colors found in keratin and are associated with structural coloring and/or melanin production [27-30]. Clusters of early filaments described in Case 1 demonstrate that fibers are anchored and growing from a basal epithelial cell matrix.
They are clearly biological and human in nature and are not implanted textile fibers. Various growth stages of fibers attached to epidermal matrices have been observed. These range from early filaments isolated or in clusters (that are only a few µm in diameter and 10 µm long) to long tangled mats (with fibers 10 µm or wider in diameter and several hundred µm long). Similar filament structures have previously been reported and photographed in MD [31]. Textile fibers have never been produced in this manner, and the suggestion that these unusual formations are manufactured textile fibers is not credible.
Longer fibers with tapered ends anchored to a cellular matrix were observed in Case 1, demonstrating filament evolution. Colored fibers were often found near larger hair follicles or appeared to have follicular bulb-like ends, suggesting an association with hair follicles and follicular keratinocytes. Our chemical studies suggest that MD filaments and
BDD fibers react to caustic agents in a manner similar to normal hair, although MD filaments appeared to be more susceptible and BDD fibers less susceptible to the caustic agents.
In preliminary studies using scanning electron microscopy, the presence of scales on a blue filament indicated that this specimen was a fine hair (D’Alba L and Shawkey MD, unpublished observation, December 2011). This finding suggests that some of the colored fibers of follicular origin may in fact be modified hairs. Differences between the keratinocytes found in the inner root sheath of hair follicles and keratinocytes found in the basal skin layer may account for the differences of location, structure, coloring and size of fibers seen in this study [32,33]. The effect of spirochetes on keratinocyte function may also play a role in altered keratin production associated with MD and BDD [22,25,26].
In conclusion, MD lesions were not caused by self-mutilation or delusions in the three cases presented here. The photographic evidence clearly demonstrates that the unusual fibers or filaments described in this study are not self-implanted textile fibers. All three patients had symptoms and laboratory findings consistent with systemic illness and
indicative of tickborne disease. Neuropsychiatric testing was normal in two cases and influenced by the disease in the third case, and all three patients were examined by a medical practitioner who confirmed the presence of fibers underneath unbroken skin compatible with a diagnosis of MD.
We have demonstrated that filaments found in MD patients have chemical, physical and immunohistological features of keratin. The presence of individual filaments attached to epithelial tissue is consistent with keratin and suggests that the filaments are produced by keratinocytes. Morgellons filaments have been photographed growing from pavement epithelial cells, and this process resembles the evolution of filaments seen in BDD.
Because BDD is a disease in which spirochetes have been identified as primary etiologic agents, and spirochetal sero-reactivity has been associated with MD, it is reasonable to assume that spirochetal infection plays an important role in MD filament production. Further immunohistological and electron microscopy studies are needed to solve the mystery of Morgellons filaments.
Conflict of Interest Statement
RBS serves without compensation on the medical advisory panel for QMedRx
Inc. He has no financial ties to the company. MJM, EHR and RBS serve without
compensation on the scientific advisory panel of the Charles E. Holman Foundation.
DGK has no conflicts to declare.
Acknowledgments
The authors thank Drs. Gordon Atkins, Robert Bransfield, Dorte Dopfer,
Alan MacDonald, Peter Mayne, Deryck Read, Matthew Shawkey, Janet Sperling,
Ginger Savely, Michael Sweeney and Randy Wymore for helpful discussion. We
thank Dr. Robert B. Allan for technical support and Lorraine Johnson for manuscript
review, and we are grateful to Harriet Bishop, Cindy Casey and Lee Laskowsky for
providing first-hand information about Morgellons disease.
References
1. Savely VR, Leitao MM, Stricker RB (2006) The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol 7: 1-5.
2. Savely VR, Leitao MM (2005) Skin lesions and crawling sensations: disease or delusion? Adv Nurse Pract 13: 16-17.
3. Middelveen MJ, Stricker RB (2011) Filament formation associated with spirochetal infection: A comparative approach to Morgellons disease. Clin Cosmet Investig Dermatol 4: 167-177.
4. Elkan D. Morgellons (2007) disease the itch that won’t be scratched. New Scientist 2621: 46-49.
5. Wymore RS (2011) Morgellons disease research; shotgun DNA analysis, PCR, microscopy and spectroscopy. Morgellons Medical Conference, Austin, Texas.
6. Pearson ML, Selby JV, Katz KA, Cantrell V, Braden CR, et al. (2012) Clinical, epidemiologic, histopathologic and molecular features of an unexplained dermopathy. PLoS ONE 7: e29908.
7. Savely VR, Stricker RB (2007) Morgellons disease: the mystery unfolds. Expert Rev Dermatol 2: 585-591.
8. Harvey WT (2007) Morgellons disease. J Am Acad Dermatol 56: 705-706.
9. Harvey WT, Bransfield RC, Mercer DE, Wright AJ, Ricchi RM, et al. Morgellons disease, illuminating an undefined illness: a case series. J Med Case Reports 3: 8243.
10. Savely VR, Stricker RB (2009) Morgellons disease: analysis of a population with clinically confirmed microscopic subcutaneous fibers of unknown etiology. Clin Cosmet Investig Dermatol 3: 67-78.
11. Cheli R, Mortellaro CM (1974) Digital dermatitis in cattle. Proc 8th Int Meet Dis Cattle, Milan, Italy 8: 208-213.
12. Vink WD, Jones G, Johnson WO, Brown J, Demirkan I, et al. ( 2009) Diagnostic assessment without cut-offs: application of serology for the modeling of bovine digital dermatitis infection. Prev Vet Med 92: 235-248.
13. Dopfer D, Willemann MA(1998) Standardisation of infectious claw diseases. Proceedings of the 10th International Symposium on Lameness in Ruminants; Lucerne, Switzerland 244-254.
14. Borgmann JE, Bailey J, Clark EG (1996) Spirochete-associated bovine digital dermatitis. Can Vet J 37: 35-37.
15. 15. Blowey RW, Sharp MW (1988) Digital dermatitis in dairy cattle. Vet Rec 122: 505-508.
16. Read DH, Walker RL, Castro AE, Sundberg JP, Thurmond MC (1992) An invasive spirochaete associated with interdigital papillomatosis of dairy cattle. Vet Rec 130: 59-60.
17. Scavia G, Sironi G, Mortellaro CM, Romusi S (1994) Digital dermatitis: further contribution on clinical and pathological aspects in some herds in northern Italy. Proc Int Symp Dis Ruminant Digit 8: 174-176.
18. Grund S, Nattermann H, Horsch F (1995) Electron microscopic detection of spirochetes in dermatitis digitalis of cattle. Zentralbl Veterinarmed B 42: 533-542.
19. Döpfer D, Koopmans A, Meijer FA, Szakáll I, Schukken YH et al. ( 1997) Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet Rec 140: 620-623.
20. Demirkan I, Carter SD, Murray RD, Blowey RW, Woodward MJ(1998) The frequent detection of a treponeme in bovine digital dermatitis by
immunochemistry and polymerase chain reaction. Vet Microbiol 60: 285-292.
21. Read DH, Walker RL(1998) Papillomatous digital dermatitis (footwarts) in California dairy cattle: clinical and gross pathologic findings. J Vet Diagn Invest 10: 67-76.
22. Gomez A, Cook N, Dopfer D, Bernardoni N, Dusick A, et al. (2011) An experimental infection model for digital dermatitis. Lameness in Ruminants Conference, New Zealand.
23. Hamajima Y, Komori M, Preciado DA, Choo DI, Morobe K et al. (2010) The role of inhibitor of DNA-binding (ID1) in hyperproliferation of keratinocytes: the pathological basis for middle ear cholesteatoma from chronic otitis media. Cell Prolif 43: 457-463.
24. Raynov AM, Choung YH, Park HY, Choi SJ, Park K (2004) Establishment and characterization of an in vitro model for cholesteatoma. Clin Exp
Otorhinolaryngol 1: 86-91.
25. Tjernlund U, Scheynius A, Asbrink E, Hovmark A (1986) Expression of HLA-DQ antigens on keratinocytes in Borrelia spirochete-induced skin lesions. Scand J Immunol 23: 383-388.
26. Gebbia JA, Coleman JL, Benach JL (2001) Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect Immun 69: 456-462.
27. Shawkey MD, Hill GE (2005) Caratenoids need structural colors to shine. Biol Lett 1: 121-124.
28. Shawkey MD, Shreekumar RP, Hill GE, Siefferman LM, Roberts SR (2007) Bacteria as an agent for change in structural plumage color: correlational and experimental evidence. Am Nat 169: S112-S121.
29. D’Alba L, Saranathan V, Clarke JA, Vinther JA, Prum RO, et al. (2011) Colourproducing ß-keratin nanofibres in blue penguin (Eudyptula minor) feathers. Biol Lett 7: 543-546. .
30. Chung WJ, Oh JW, Kwak K, Lee BY, Meyer J, et al. (2011) Biomimetic selftemplating supramolecular structures. Nature 478: 364-368.
31. Charles E. Holman Foundation (2012) Accessed February 16.
32. Rugg EL, Leigh IM (2004) The keratins and their disorders. Am J Med Genet C Semin Med Genet 131C: 4-11.
33. Moll R, Divo M, Langbein L (2008) The human keratins: biology and pathology. Histochem Cell Biol 129: 705-733.
Filament formation associated with spirochetal infection:
a comparative approach to Morgellons disease
October 4, 2012 http://www.morgellons-uk.net/?p=824
Abstract
Bovine digital dermatitis is an emerging infectious disease that causes lameness, decreased milk production, and weight loss in livestock. Proliferative stages of bovine digital dermatitis demonstrate keratin filament formation in skin above the hooves in affected animals. The multifactorial etiology of digital dermatitis is not well understood, but spirochetes and other coinfecting microorganisms have been implicated in the pathogenesis of this veterinary illness.
Morgellons disease is an emerging human dermopathy characterized by the presence of filamentous fibers of undetermined composition, both in lesions and subdermally.
While the etiology of Morgellons disease is unknown, there is serological and clinical evidence linking this phenomenon to Lyme borreliosis and coinfecting tick-borne agents. Although the microscopy of Morgellons filaments has been described in the medical literature, the structure and pathogenesis of these fibers is poorly understood. In contrast, most microscopy of digital dermatitis has focused on associated pathogens and histology rather than the morphology of late-stage filamentous fibers.
Clinical, laboratory, and microscopic characteristics of these two diseases are compared.
Morgellons Disease: A Chemical and Light Microscopic Study
http://www.omicsonline.org/2155-9554/2155-9554-3-140.pdf
Morgellons disease, illuminating an undefined illness: a case series
http://www.biomedcentral.com/content/pdf/1752-1947-0003-0000008243.pdf
William T. Harvey 1*,
Robert C. Bransfield 2,
Dana E. Mercer 3,
Andrew J. Wright 4,
Rebecca M. Ricchi 5 and
Mary M Leitao 6
Addresses:
1 Preventive Medicine, Colorado Springs, CO 80949, USA,
2 Psychiatry, Red Bank, NJ, 07701, USA,
3 Veterinary Medicine, Fulton, TX, 78358, USA,
4 General Practitioner, Bolton BL1 4QR, UK,
5 Adult Medicine, USAFA, CO, 80840, USA and
6 BS (Biology), Guilderland, NY 12084, USA
Email:
WTH* - idmed99@aol.com
RCB - bransfield@comcast.net
DEM - drdana@horizonvetclinic.net
AJW - drajw2003@yahoo.co.uk;
RMR - rebecrn@comcast.net
MML - MaryL@Morgellons.org
* Corresponding author
Received: 28 November 2008
Accepted: 17 March 2009
Published: 1 July 2009 Journal of Medical Case Reports 2009, 3:8243 doi: 10.4076/1752-1947-3-8243
This article is available from: http://jmedicalcasereports.com/jmedicalcasereports/article/view/8243
© 2009 Harvey et al; licensee Cases Network Ltd.
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract Introduction:
This review of 25 consecutive patients with Morgellons disease (MD) was undertaken for two primary and extremely fundamental reasons. For semantic accuracy, there is only one “proven” MD patient: the child first given that label. The remainder of inclusive individuals adopted the label based on related descriptions from 1544 through 1884, an internet description quoted from Sir Thomas Browne (1674), or was given the label by practitioners using similar sources.
Until now, there has been no formal characterization of MD from detailed examination of all body systems.
Our second purpose was to differentiate MD from Delusions of Parasitosis (DP), another “informal” label that fit most of our MD patients. How we defined and how we treated these patients depended literally on factual data that would determine outcome. How they were labeled in one sense was irrelevant, except for the confusing conflict rampant in the medical community, possibly significantly skewing treatment outcomes.
Case presentation:
Clinical information was collected from 25 of 30 consecutive self-defined patients with Morgellons disease consisting of laboratory data, medical history and physical examination findings. Abnormalities were quantified and grouped by system, then compared and summarized, but the numbers were too small for more complex mathematical analysis. The quantification of physical and laboratory abnormalities allowed at least the creation of a practical clinical boundary, separating probable Morgellons from non-Morgellons patients. All the 25 patients studied meet the most commonly used DP definitions.
Conclusions:
These data suggest Morgellons disease can be characterized as a physical human illness with an often-related delusional component in adults. All medical histories support that behavioral aberrancies onset only after physical symptoms. The identified abnormalities include both immune deficiency and chronic inflammatory markers that correlate strongly with immune cytokine excess.
The review of 251 current NLM DP references leads us to the possibility that Morgellons disease and DP are grossly truncated labels of the same illness but with the reversal of the cause-effect order. Further, the patients’ data suggest that both illnesses have an infectious origin.
Introduction
The term “Morgellons disease” first publicly appeared on the Internet in 2002. The “index” case was the first modern case to which that label was appended: a sick child whose physical signs and symptoms were collectively unrecognized as an entity at local and regional medical facilities.
As the child’s illness persisted without recognition or resolution, the unaffected parent sought similar illness descriptions from historic medical references, eventually settling on “The Morgellons”, a label given to childhood cases described in France in 1674 by Sir Thomas Browne [1]. His description was limited to its dermal components: hair-like extrusions and sensations of movement. Ettmuller, a physician, produced the only known drawings of The Morgellons in 1682 and considered these dermal “filaments” a parasitic infestation [1].
Numerous isolated descriptions of similar phenomena were found by Kellett from 1544 through 1884 that began as a pediatric-only illness identified by posterior torso “bristles” but also included extreme agitation, seizures, “wasting” and death (characteristics not seen in the 21st century).
By the early 1600s, the illness was thought to be caused by the parasite Dracunculus (later called Dracontia, then pilaris affectio) and “cured” by filament (agent) removal from the skin.
The worm theory was considered debunked by 1715 when the esteemed microscopist, Leuvenhoeck, pronounced the bristles “inanimate”.
From this point forward to 1884, however, the illness shifted toward comedones, facial regions and to adults. And Demodex folliculorum was considered a frequent occupant of comedones, again supporting a “parasite” concept.
The name “Morgellons disease” was thus created in 2002, as a practical “place holder” because of its dermal similarity to The Morgellons described by Browne in particular (Browne’s The Morgellons label evolved further over three centuries into the words, “Masquelon”, then Masclous dicti and Les Crinons, shifting to Comedones after 1800).
The new label also provided an alternative to differentiate a clearly, non-delusional child from many decades of clinical use of the label Delusions of Parasitosis [2-10].
This first set of actions would eventually raise one of the major philosophical questions of modern medicine:
Does the practice of “evidence-based medicine” come from peer-reviewed literature, or from the actual patient? Information from what was later to become the Morgellons Research Foundation (MRF) website initially provided a broader look at the believed Morgellons disease symptoms by allowing patient registrants to record their symptoms and signs.
Clearly, most of the 21st century patients did not fit the descriptions from 1500-1800.
The MRF website provided a glimpse of the current global prevalence of those considering they are affected by this phenomenon. On January 2009, there are 13,000+ family registrants from all U.S. states and from 15 other countries.
[http://www.morgellons.com/] Given the growing use of the label Morgellons disease, characterizing the illness using verifiable clinical data seemed to be the imperative first step for clinicians, particularly to separate these patients from those two to five centuries before presentation.
Until 2007, Morgellons and DP cases were often seen in isolation, interfering with credible Case Definitions. Assembling even this small number of candidate patients into a series with verified data points, should at least improved diagnosis consistency.
Case Series Data Collection
The study protocol was simply a collection of clinical data obtained from new Morgellons disease patients at one clinic in a two-hour session between September 2006 and July 2007.
Patients were not solicited but came self-diagnosed.
Practice size-limited the original patient number to 30 adults.
The final number was 25 after filtering for consistency and data errors.
Each clinical session included a detailed history, review of systems and a physical examination emphasizing dermal, neurological, endocrine and psychiatric systems.
Expanded laboratory tests included a blood count (CBC), a metabolic panel (CMP) and others to evaluate major organ system function to characterize immune status, detect inflammation and search for an initial and limited number of possible infectious pathogens suggested by several veterinarians [personal communication].
Subjective screening criteria derived from earlier clinical encounters were first applied to improve patient selection consistency (Table 1).
The screened data were then entered into 492 fields on each patient, 407 fields of which contained useful data points. This provided 10,175 total parameters for analysis.
Case Series Initial Data Summary
The first-level summary of raw data is in the Appendix, Tables A, B and C. Categories devoid of entries had no significant findings.
Table 1.
Initial Patient Screening Criteria
1. Patient convinced of chronic parasite infestation.
2. Primary patient illness focus must be either on dermal sensations or on “never-before-seen” material thought to be extruded from his or her body, even if this was not the most debilitating symptom or sign. The material must have been described as “fibers”, “fiber-like”, or “filaments”.
3. Chronic pruritis (itching) must have been present for at least six months.
4. The patient must have at least two chronic, unhealed skin lesions recorded by a clinician, regardless of whether excoriation was suspected.
5. Prior diagnosis of Delusions of Parasitosis or bipolar illness would not exclude the patient as a candidate.
6. Patients experiencing delusional states common in withdrawal from drugs such as opiates and patients in organic brain states, were excluded.
7. The illness must have had a life-altering effect on the patient.
8. The patient must be an adult and had experiencing symptoms for more than six months.
9. A healthy pre-morbid period in the patient’s life was acceptable.
A. Demographic Information
Male and female rates were the same for those who believed they had Morgellons disease.
Age spread, geographic spread and gender neutrality were large. These data suggest Caucasian bias that did not appear in data from other countries [11].
B. Illness History
Rural residence or recent rural travel was common.
Emotional stress was not relevant, nor was season of onset. Physical stress was a common precursor.
Full onset typically required one to several months from first symptom.
C. Past Medical History
Prior psychiatric diagnoses in more than half. The most common diagnosis was bi-polar illness.
D. Social History
Significantly reduced exercise capacity was highly prevalent although non-specific as an illness marker. Recent exposure to unhygienic settings (Third world countries, sewage systems and land fills) correlated strongly with illness onset.
E. Systems Information from physical examination and History of Present Illness General:
Weight gain after illness onset averaged to 33 pounds.
High levels of fatigue and reduced exercise capacity were eventually present in 80% of patients.
Recurrent fever by thermometry was noted by 50% of patients.
Dermatological:
A typical phrase used by most patients was similar to: Extruding and moving skin “parasites” (filaments, “fibers”, “spheres”) generating uncomfortable lesions. Patients stated “filament” appearance was intermittent and consistent with identifying in less than one in three patients on examination. The sensation of movement, however, was denied by up to 50% of those experiencing filament extrusions.
About 70% had regular appearance of painful shallow skin ulcers, but most could easily separate excoriations (scratching) they had generated from spontaneous ulcers.
Numerous micro-angiomas (0.5 to 3.0 mm in diameter) were found by examination on 72% of patients. This phenomenon is a known hallmark of Bartonellosis, although our patients had completely negative Bartonella serology [12,13].
Many patients noted that angiomata appearance directly paralleled illness onset and progression.
Central Nervous System:
This group experienced a high rate of headaches, visual aberrancies, tinnitus, and short-term memory deficits.
Emotional lability was present in more than half, typically manifest intermittently.
Cardiovascular:
Patients often reported orthostatic intolerance and frequent arrhythmias (type undetermined) regardless of age.
Rhythm and mild cardiac auscultation abnormalities (valvular) were commonly found on vital sign determination.
Pulse was high (>72) in virtually all.
Psychiatric:
Strikingly, most patients in this study (23 out of 25) had prior psychiatric diagnoses (most determined by specialists) as follows:
11 out of 25 bipolar disease;
7 out of 25 Adult ADD;
4 out of 25 Obsessive Compulsive Disorder (OCD);
and 1 out of 25 Schizophrenia.
Although overlap occurred in 5 cases, only the primary psychiatric diagnosis was tabulated [14].
In each case, medical records indicated that the dermal symptoms and signs preceded or occurred simultaneously with the onset of emotional signs, with an emotionally “normal” time in each patient’s life prior to Morgellons disease.
Endocrine:
Eight patients (32%) had prior diagnoses of Hashimoto’s Thyroiditis. [The U.S. adult prevalence rate of Hashimoto’s Thyroiditis is 0.56%. The rate is based on 1996 statistics: 1,490,371 adults with Hashimoto’s Thyroiditis per U.S. population of 264,162,000. (Reference: Rose and Mackay, 1998, The Autoimmune Diseases, Third Edition)] Half had past evidence of Hypercalcemia (intermittent), of which 3 had definite parathyroid adenomas, later surgically excised with incomplete improvement.
Fasting insulin levels were elevated in 100% who were tested for it (6 out of 25), as were CRH levels (also 6 out of 25 tested).
Other:
Recurrent cough and dyspnea were common as GI and urinary symptoms, but none had a recent medically determined etiology.
F. Vital Signs
The most Consistent markers in this group were low core temperature and high resting heart rate, affecting all 25 patients.
G. Laboratory Data
CBC aberrancies were common but often intermittent. They included abnormal variable RBC indices, occasional low-grade anemia, low white cell count, and high monocyte count.
Other abnormalities included low Natural Killer cell (CD56 + CD16) number and percentage, high or high-normal insulin level (in all tested) and intermittent elevation of serum calcium, globulin level, and A/G ratio. Sedimentation rate was mid-range “normal” or lower with only one ANA positive. Antidouble stranded DNA antibody level was negative in all tested. Occasionally IgG subclasses 1 and 3 were low. Commonly elevated markers supporting chronic inflammation included C-reactive protein and TNF-alpha.
Other occasionally abnormal laboratory parameters present in chronic inflammation or infection included IFN-gamma, Homocysteine and serum Leptin. Most patients showed serologic evidence of infection (antibodies) with one or more unexpected potentially pathogenic microorganisms despite testing for only a few species.
Data Summary
Following is the concluding summary of collected data from all patients, including pathological mechanisms suggested by the pattern(s) of these data anomalies.
Morgellons disease was often preceded by exposure to unhygienic conditions and appears first as skin abnormalities and discomfort.
Illness onset appears with moderately rapid transition (weeks) from healthy to unhealthy, including “emotional discomfort”.
These gravid Morgellons females had an extraordinarily high miscarriage rate.
The most common physical abnormalities found in this series include:
1) Numerous “senile angiomas” on the trunk, head and limbs of many;
2) Recurrent fever;
3) Awareness of itching, crawling, stinging or biting. When present, patients describe a circadian tempo to the symptoms; some occurring solely at night. Itching of unbroken skin specifically appeared to precede all other skin symptoms;
4) Unidentified objects (called “filaments” or “granules”) “extrude” uncomfortably from unbroken skin or skin lesions;
5) Painful ulcer-like concave, circular skin lesions with distinct border;
6) Excoriations adjacentto but separate from ulcerations were common;
7) Dermal symptoms were the central focus of discomfort for most patients.
8) Multiple organ system symptoms often appeared within the first six months of illness onset.
The most common systems affected were the central and peripheral nervous systems, autonomic nervous system then endocrine, cardiovascular, and pulmonary systems.
All blood pressures were low and all resting pulses were high.
Routine laboratory tests were often inconsistent and varied both positively and negatively, but within range more frequently than out of range.
Common abnormalities were NK cell numbers and percentages (low), and fasting insulin levels (very high).
Occasionally abnormal were RBC indices, hematocrit, WBC count (low), monocyte count (high), serum calcium (high), globulin level (high), and A/G ratio (high). Contradictory autoimmunity data was frequently noted.
Some expected inflammation tests such as sedimentation rate and Anti-double stranded DNA antibody tests were negative, while C-reactive protein and TNF-alpha were routinely high. IFN-gamma, Homocysteine and Leptin were also elevated.
There was a high prevalence of transient “autoimmune” diseases such as Hashimoto’s Thyroiditis, hyperparathyroidism and adrenal-cortical hypofunction.
Following is a second level summary of the Morgellons data (Table 2).
This is intended as a simplistic tool for clinical use. Conclusion Proposed Characterization of Morgellons Disease
The authors conclude that Morgellons disease is a multi-systemic illness that has been presumed as a delusional phenomenon for decades as its most obvious and disconcerting manifestations resembled actual (but “unverified”) parasite infestation as well as various psychopathologies.
However, using recent technology and even a modicum of consistently obtained physical data supports that Morgellons manifest as a skin phenomenon, an immune deficiency state and a chronic inflammatory process.
Since infectious agents can initiate and maintain chronic diseases, the behavioral and other CNS manifestations here are more likely effect than cause [18]. We suggest that the Morgellons label be considered to displace any label suggesting delusion as the primary cause of this phenomenon.
The term “Morgellons disease” came into being in the 21st century in an attempt to identify an illness for which no name existed. The entry of this new label into a phenomenon of extreme controversy may at first appear to further that controversy. Typical of the evolution of medical nomenclature, however, the problem may always have been with semantics; in particular the use of assumptions unsupported from failure to investigate the total physical patient [19].
The “index” case that failed to fit similar earlier diagnostic labels was seen as “different” principally because its observer looked beyond the presumed signs and symptoms of the truncated “look-alike” phenomenon labeled “Delusions of Parasitosis”.
Trabert’s review of 1,300+ cases makes clear that only a fraction of the total signs of that presumed illness were used to create the DP diagnostic framework [14,20].
Our attempt to gather as much physical evidence on Morgellons patients as possible was based on the extremely large number of abnormal physical signs among those we evaluated earlier. We gathered as many clinical parameters as possible (within the fiscal constraints of “today’s” medicine) in order to see whether the abnormalities among them were consistent and if so, whether their pattern was explanatory. The unfolding mechanism strongly suggests a chronic infectious process.
Table 2.
Primary Abnormal Findings
1. The large age spread, geographic spread and gender neutrality among patients suggest broad human susceptibility to the illness. 2. Rural abode or exposure to unhygienic conditions (third world travel or simply soil exposure) may be risk factors.
3. Onset rate is moderately rapid, without recognizable prodrome, commonly preceded by a healthy state.
4. Once ill, exercise capacity is significantly reduced.
5. The illness is common among family members and close associates, both related genetically and unrelated (such as spouses).
6. Most patients experience weight gain after disease onset.
7. Micro-angiomas appear rapidly on skin after illness onset in most.
8. Fever is recurrent in at least half of those affected.
9. The first illness sign may be the sudden appearance of persistent itching. Ulcerative lesions follow in some cases.
10. Once dermal symptoms begin, patients experience extrusion of unfamiliar material described variously as filamentous, “fuzz balls”, black or white “flecks” or “rice grains”.
11. Numerous CNS effects occur, that includes bizarre cranial nerve phenomena, anxiety and emotional sequelae. The former tend to be transient.
12. Numerous Peripheral Nervous System findings appear after illness onset. Unlike CNS effects, these are serious, permanent and progressive, and include sensory and motor nerves.
13. All Morgellons have elevated heart rate (>72 BPM) and low body temperature by oral thermometry (<97.5 degrees F).
14. Orthostatic intolerance is intermittent but common in most.
15. Most have some formally diagnosable emotional illness that begins with or becomes apparent after Morgellons disease onset. 16. Endocrine abnormality number and type is higher than background. Most common are Diabetes Type II, Hashimoto’s Thyroiditis, hyperparathyroidism and adrenal hypofunction.
17. Most have elevated fasting insulin levels accompanied by elevated TNF-alpha (insulin receptor blocker) [15,16].
18. Common clinical laboratory abnormalities include: a. RBCs have abnormal morphology. On manual examination, RBCs were non-discoid, varied in color and size. b. Natural Killer Cell (CD 56 + CD 16) number and function are very low in most. c. A/G ratio and globulin level are frequently elevated. d. Sedimentation Rate and ANA are extremely low despite other common autoimmune-like conditions. e. Elevated cytokines include: TNF-alpha, IFN-gamma, IL-6, C Reactive Protein, Homocysteine and serum Leptin.
19. Despite no fact-based Case Definition of Delusions of Parasitosis (DP), each of our 25 patients could have been given such an illness label as well. As pointed out by Trabert in a meta-analysis of 1,223 DP cases and others, most DP data were taken from isolated cases. Psychiatric-skewed labels were common such as “psychocutaneous disease”, “acarophobia” and “monosymptomatic hypochondriasis” with no serious search for consistent physical abnormalities or microscopic parasitic agents [17].
The specific agent candidates will not be addressed further until evidence of their presence is available and their presence can explain the signs and symptoms we now find in all Morgellons patients. There remains considerable work to do in collecting more data from these patients to create a credible Morgellons disease Case Definition. We submit that the same holds true for Delusions of Parasitosis patients [3]. Much of that work may be now under way by the U.S. Centers for Disease Control and Prevention (CDC) through contract with Kaiser Permanente’s Northern California Division of Research. [http://www.cdc.gov/unexplaineddermopathy/]
Meanwhile, the consistent abnormal findings in the data above may be used to improve clinical diagnosis and possibly initial treatment in current patients. Perhaps of considerable importance to a journal dedicated to Case Reports is what the juxtaposition of Morgellons disease and Delusions of Parasitosis suggests.
As noted by Trabert in his meta-analysis, creating a Case definition primarily from isolated cases allows uncontrolled use of assumptions that vary considerably in order to keep an unresolved conclusion constant. Where many cases are used, consistency of similar data forces a far more valid and consistent conclusion [14,20].
Until the machinery of science is in full gear and provides understanding of this phenomenon, simply “paying attention”, maintaining skepticism, practicing a simple physical exam and using commercial laboratories judiciously must suffice.
Once the breadth and severity of what we are encountering is understood, the resources and motivation for its solution should come. When sufficient, we anticipate the framework of several medical specialties may be modified.
Consent
Written informed consent statements were obtained from the patients for publication of this case report. The copies of the written consent are available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they do not have any competing interests.
Authors’ contributions
WH crafted the submission in its final form, including the cover letter, abstract, title page and references, drafted the summary and conclusions, and submitted the package to JMCR. RB assisted with the completion of the entire submission and was responsible for all sections addressing psychiatric elements of the illness, particularly references to Delusions of Parasitosis.
More will follow in paper #2. DM was responsible for all references to known parasite species and all conclusion statements considering parasitosis. AW analyzed all micrographs from several fluid and tissue sources for pathogen identification and contributed data from his own pool of anonymous UK patients for context. RR obtained medical histories and systems review from all series patients and applied her FBI forensics scrutiny training to complete a second physical examination on each patient. ML is the creator of the current Morgellons label and concept, and until characterized systematically, its sole “authority”. She created the Morgellons registration website, and modified this submission for consistency with the 1300+ site registrants.
All authors read and approved the final manuscript.
Acknowledgements None.
References
1. Kellett C: Sir Thomas Browne and the Disease called the Morgellons. Annals of Medical History 1935, 7:467-479.
2. Robles DT et al.: Delusional disorders in dermatology: a brief review. Dermatol Online J 2008, 14:2.
3. Berrios GE: Delusional parasitosis and physical disease. Compr Psychiatry 1985, 26:395-403.
4. Szepietowski JC et al.: Delusional parasitosis in dermatological practice. J Eur Acad Dermatol Venereol 2007, 21:462-465.
5. Nicolato R et al.: Delusional parasitosis or Ekbom syndrome: a case series. Gen Hosp Psychiatry 2006, 28:85-87.
6. Aw DC, Thong JY, Chan HL: Delusional parasitosis: case series of 8 patients and review of the literature.
Ann Acad Med Singapore 2004, 33:89-94.
7. Koo J, Gambla C: Delusions of parasitosis and other forms of monosymptomatic hypochondriacal psychosis.
General discussion and case illustrations. Dermatol Clin 1996, 14:429-438.
8. Johnson GC, Anton RF: Delusions of parasitosis: differential diagnosis and treatment. South Med J 1985, 78:914-918.
9. Amato Neto et al.: Ekbom syndrome (delusory parasitosis): ponderations on two cases. Rev Inst Med Trop Sao Paulo 2007, 49:395-396.
10. Bouree P, Benattar B, Perivier S: Ekbom syndrome or delusional parasitosis. Rev Prat 2007, 57:585-589.
11. Savely VR, Leitao MM, Stricker RB: The mystery of Morgellons disease: infection or delusion? Am J Clin Dermatol 2006, 7:1-5.
12. Podsiadly E, Chmielewski T, Tylewska-Wierzbanowska S: Bartonella henselae and Borrelia burgdorferi infections of the central nervous system. Ann N Y Acad Sci 2003, 990:404-406.
13. Brouqui P et al.: Ectoparasitism and vector-borne diseases in 930 homeless people from Marseilles. Medicine (Baltimore) 2005, 84:61-68.
14. Aaron LA, Buchwald D: A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001, 134:868-881.
15. Holden RJ, Pakula IS, Mooney PA: Tumor necrosis factor-alpha: a continuum of liability between insulin-dependent diabetes mellitus, non-insulin-dependent diabetes mellitus and carcinoma (review). Med Hypotheses 1999, 52:319-323.
16. Nishimura M et al.: Role of tumor necrosis factor-alpha in insulin sensitivity and effect of low protein diet on the TNFalpha response in patients with diabetic renal failure. Nippon Jinzo Gakkai Shi 1997, 39:740-745.
17. Trabert W: Epidemiology of delusional ectoparasitic infestation. Nervenarzt 1991, 62:165-169.
18. Ewald PW: The evolution of virulence. Sci Am 1993, 268:86-93.
19. Ruchlis H: Clear Thinking: a practical introduction. 1st edition. 1990, Buffalo, NY: Promethius Books, 1990:271.
20. Trabert W: 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology 1995, 28:238-246.
APPENDIX TABLES OF RESULTS NOTE:
These tables are a first level summary of the complete basic data set except for physical examination findings that are summarized above. Although subjectivity has been introduced, we have attempted to keep it separate as footnotes.
Table A.
Basic Background Data
Demographics Countries of origin: U.S., U.K., Canada, Japan
Male: Female ratio approximates 50:50
Age range: 10-75 years
24/25 were Caucasian
Urban: Rural ratio = 50:50
Past medical history
11 out of 25 were diagnosed with bipolar disease
7 out of 25 were diagnosed with adult ADD
1 out of 25 was diagnosed with schizophrenia
Common childhood illnesses were absent regardless of vaccination status
19 out of 25 had been given general anesthesia at least once
9 out of 25 had prior orthopedic surgery
6 out of 25 had other prior surgery for chronic inflammatory diseases (appendix, gallbladder)
Illness History
Time from initiation to full onset: several months
Exposure to unhygienic conditions occurred several days to several weeks prior to illness onset
Patients claimed relative healthiness prior to Morgellons (as initially described) onset;
No consistent recognized prodrome heralded onset of the illness
Allergy symptoms were rare
Activating stressor event was present in 1 out of 3 of cases
Social History
50% were married/ 50% single
28% were smokers
None routinely exercised
Highest relevancy results.
Findings that may help to isolate, identify, and define Morgellons disease.
Other data.
1 This sample was not adequate to determine whether race is a factor. The high percentage of Caucasian patients was likely related to clinic location. Earlier and subsequent data of larger numbers from more heterogeneous regions suggest that race is likely not a risk factor.
2 The urban to rural ratio may be significant. Although only 20% of people live in rural areas, 50% of the patient samples were in rural regions near the onset of their illness, lending support to the hypothesis that exposure to unhygienic conditions or animals may be a risk factor.
Table B.
Review of Systems
Dermatologic
24% of patients had dark filaments visibly protruding from the skin
17 out of 25 had frequent skin eruptions/rashes
Just over 50% experienced no movement sensations
Only 50% itched regularly 72% has angiomata appear
Central Nervous System
Over half had frequent headaches
Over half had unstable visual acuity including double vision (10 out of 25)
10 out of 25 reported visual “flashes” (not characterized) in either daylight or dark
Emotional liability in half of patients
1/3 reported frequent dizziness
1/3 reported permanent hearing loss (degree not determined)
40% reported tinnitus
20 out of 25 reported frequent occurrence of forgetfulness
12 out of 25 had persistent regional skin numbness
6 out of 25 had persistent tremors
Metabolic Average weight gain after onset:
33 lbs
20 out of 25 experienced regular high levels of fatigue
25% were intolerant to cold and
25% intolerant to heat (some overlap, but degree undetermined)
10 out of 25 reported sweating inappropriately (either under- or over-sweating)
Immune
12 out of 25 had cyclical fever
18 out of 25 reported numerous angiomas verified by examination
10 out of 25 reported many “colds” annually
8 out of 25 reported “chronic sinusitis”
4 out of 25 reported frequent sore throats over a period of years
2 out of 25 reported frequent toothaches
Endocrine
8 out of 25 had prior diagnosis of Hashimoto’s Thyroiditis
50% had hypercalcemia,
3 of whom had parathyroid adenomas
Fasting insulin levels were elevated in all tested (6 out of 25)
Corticotrophin Releasing Hormone (CRH) levels were elevated in all tested
Musculo-skeletal
15 out of 25 reported frequent neck pain
10 out of 25 had persistent limited neck movement
22 out of 25 had chronic musculoskeletal pain,
without arthritis Pain was constant and incapacitating in 1/3 of patients
Gastro-intestinal
50% had constipation or diarrhea, or both (overlap was common)
25% had chronic nausea
Pulmonary Persistent cough (non-productive, non-wheezing) in 9 out of 25
Persistent dyspnea in 7 out of 25
Emotional
Half reported “anxiety”
Half reported frequent mood swings
Half reported frequent depression
Other
Urine stream control problems in 6 out of 25
1 Less than half of the 25 patients studied reported the presence of filaments, and as a result, the authors did not search for filaments on all patients.
2 Average weight gain in male and female patients combined. Time period for weight gain undetermined.
Table C.
Vital Signs and Laboratory Data Lab Parameters Laboratory Values
Vital Signs
Average temp 97.51° F
Average pulse 85.32 BPM Laboratory Data
Unidentified lymphatic filamentous clusters were found in 4 patients
50% were positive for Borrelia burgdorferi sensu lato either by CDC Western Blot (WB) criteria, EUCALB WB (IgM P31 and 34 added), or IFA
4 patients were seropositive for Babesia microti 92% were seropositive for Chlamydia pneumonia (Chp)
1 Suggests Borrelia may contribute to some cases of Morgellons disease.
2 Suggests Babesia is uncommon in most Morgellons disease cases.
3 Suggests Chlamydia pneumonia (Chp) is one candidate for the initial etiology of Morgellons disease via generation of an immunodeficiency state.
4 Lab tests were drawn at 6,100 feet MSL (above mean sea level).
5 RBC characteristics were adjusted for elevation. All indices should be “normal.”
6 Lower than regional mean of 66%.
7 Slightly higher than regional mean of 28%.
8 Much higher than regional mean of 3.0%.
9 Although a broad “normal” range exists for NK number, our clinical experience supports that consistently healthy individuals who rarely experience viral syndromes have NK # > 200. Our patients’ range is not infrequently below 10. This suggests one component of an immune deficiency state.
10 Well above regional mean of 84.
11 Well above regional mean of 0.6.
12 Although clearly “within range” at 6,100 ft. MSL, the mean of more than 100 similar patients tested near sea level (2001-2004) was 18 (21-28 anticipated). As low CO2 level translates to low serum bicarbonate level, this suggests required buffering to lower acidity. Although there are several causes of elevated serum acidity, many of these patients underwent overnight studies for sleep apnea that revealed consistent low breathing rate raising PCO2. No such formal test was included in testing these patients although we believe such testing would be highly revealing.
13 Despite this average being satisfactory, 7 of the 25 patients tested were between 10.0 and 10.6. Three of 25 patients were found to have parathyroid adenomas.
14 Greater than 5.0 in 9 of 25.
15 The converse of immune deficiency, however, suggests that a possible chronic infectious state may have been operant that could intermittently activate humoral immunity.
16 Commonly above 2.0. As globulin is elevated and albumin is clearly not low, the ratio elevation is driven somehow by the albumin. This is presently a paradox.
17 Average is high normal and well above the regional mean of 68. Suggests skeletal involvement in the disease, although liver involvement is possible. Most Morgellons patients have shown evidence of osteopenia or osteoporosis when randomly tested despite age. Bone density was not determined in these 25 patients. We believe doing so would be valuable.
18 Argues against an autoimmune role, particularly as 5 out of 25 were 0, and all were less than 20.
19 Despite this normal finding, Hashimoto’s Thyroiditis is common in larger similar groups of Morgellons. Further evaluation of the HP axis and the endocrine system in general should be included in any future studies.
20 Currently used to assess cardiac risk in three stages. In this context, we are assessing chronic inflammation. This average supports the presence of a persistent inflammatory process that parallels physical evidence of vasculitis.
21 Five >150, suggests cytokine activation and possible inflammatory effect.
22 High-normal. 11 out of 25 >3.6, suggests cytokine activation and possible inflammatory effect.
23 Definite elevation in 92%. Most commonly corresponds to insulin receptor blockade and to chronic inflammatory effects.
24 Elevation of Transforming Growth Factor beta (TGF-beta) can occur when pathological events diminish its protective growth modulating effects on various tissues. This test result parallels evidence of excessive growth processes observed in these patients such as numerous angiomata, skin tags, nevi, and regions of increased epidermis density.
25 Consistently elevated in most male and female study patients. Parallels the gain in body fat and angiogenesis experienced by most following illness onset.
DEFINITIONS
AGROBACTERIUM
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Agrobacterium
Agrobacterium is a genus of Gram-negative bacteria established by H. J. Conn that uses horizontal gene transfer to cause in plants. Agrobacterium tumefaciens is the most commonly studied species in this genus. Agrobacterium is well known for its ability to transfer DNA between itself and plants, and for this reason it has become an important tool for genetic engineering. The Agrobacterium genus is quite heterogeneous. Recent taxonomic studies have reclassified all of the Agrobacterium species into new genera, such as Rueguris, Pseudorhodobacter and Stappia, but most species have been reclassified as Rhizobium species.
CHEMTRAIL
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Chemtrail_conspiracy_theory
The term specifically refers to aerial trails allegedly caused by the systematic high-altitude release of chemical substances not found in ordinary contrails, resulting in the appearance of characteristic sky tracks. Supporters of this conspiracy theory speculate that the purpose of the chemical release may be for solar radiation management, population control, weather control, biological warfare/chemical warfare and that these trails are causing respiratory illnesses and other health problems.
HORIZONTAL GENE TRANSFER
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Horizontal_gene_transfer
Horizontal gene transfer (HGT) refers to the transfer of genes between organisms in a manner other than traditional reproduction. Also termed lateral gene transfer, it contrasts with vertical transfer, the transmission of genes from the parental generation to offspring via sexual or asexual reproduction. HGT has been shown to be an important factor in the evolution of many organisms, including bacteria, plants and humans.
Horizontal gene transfer is the primary reason for bacterial antibiotic resistance and in the evolution of bacteria that can degrade novel compounds such as human-created pesticides. This horizontal gene transfer often involves plasmids. Genes that are responsible for antibiotic resistance in one species of bacteria can be transferred to another species of bacteria through various mechanisms (e.g., via F-pilus), subsequently arming the antibiotic resistant genes' recipient against antibiotics, which is becoming a medical challenge to deal with. This is the most critical reason that antibiotics must not be consumed and administered to patients without appropriate prescription from a medical physician.
Most thinking in genetics has focused upon vertical transfer, but there is a growing awareness that horizontal gene transfer is a highly significant phenomenon and amongst single-celled organisms perhaps the dominant form of genetic transfer. Artificial horizontal gene transfer is a form of genetic engineering.
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Agrobacterium
Agrobacterium is a genus of Gram-negative bacteria established by H. J. Conn that uses horizontal gene transfer to cause in plants. Agrobacterium tumefaciens is the most commonly studied species in this genus. Agrobacterium is well known for its ability to transfer DNA between itself and plants, and for this reason it has become an important tool for genetic engineering. The Agrobacterium genus is quite heterogeneous. Recent taxonomic studies have reclassified all of the Agrobacterium species into new genera, such as Rueguris, Pseudorhodobacter and Stappia, but most species have been reclassified as Rhizobium species.
CHEMTRAIL
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Chemtrail_conspiracy_theory
The term specifically refers to aerial trails allegedly caused by the systematic high-altitude release of chemical substances not found in ordinary contrails, resulting in the appearance of characteristic sky tracks. Supporters of this conspiracy theory speculate that the purpose of the chemical release may be for solar radiation management, population control, weather control, biological warfare/chemical warfare and that these trails are causing respiratory illnesses and other health problems.
HORIZONTAL GENE TRANSFER
From Wikipedia, the free encyclopedia
http://en.wikipedia.org/wiki/Horizontal_gene_transfer
Horizontal gene transfer (HGT) refers to the transfer of genes between organisms in a manner other than traditional reproduction. Also termed lateral gene transfer, it contrasts with vertical transfer, the transmission of genes from the parental generation to offspring via sexual or asexual reproduction. HGT has been shown to be an important factor in the evolution of many organisms, including bacteria, plants and humans.
Horizontal gene transfer is the primary reason for bacterial antibiotic resistance and in the evolution of bacteria that can degrade novel compounds such as human-created pesticides. This horizontal gene transfer often involves plasmids. Genes that are responsible for antibiotic resistance in one species of bacteria can be transferred to another species of bacteria through various mechanisms (e.g., via F-pilus), subsequently arming the antibiotic resistant genes' recipient against antibiotics, which is becoming a medical challenge to deal with. This is the most critical reason that antibiotics must not be consumed and administered to patients without appropriate prescription from a medical physician.
Most thinking in genetics has focused upon vertical transfer, but there is a growing awareness that horizontal gene transfer is a highly significant phenomenon and amongst single-celled organisms perhaps the dominant form of genetic transfer. Artificial horizontal gene transfer is a form of genetic engineering.

chemtrails

Agrobacterium tumefaciens
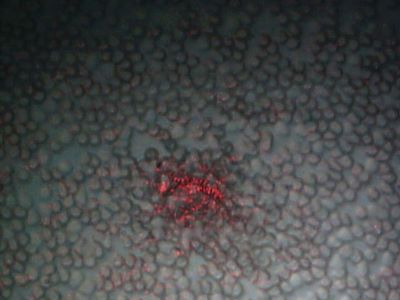
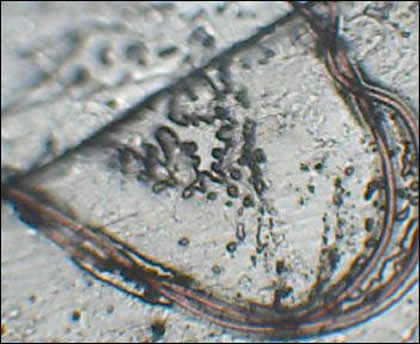
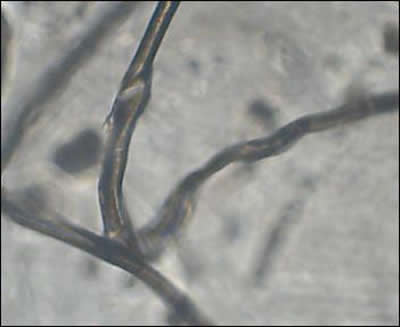
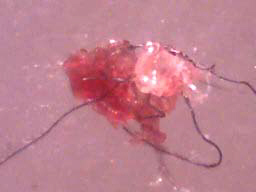
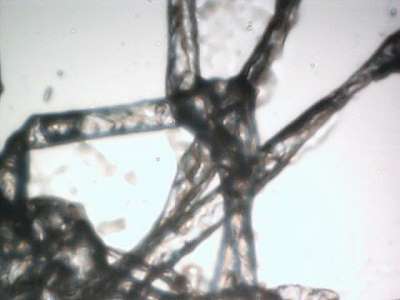
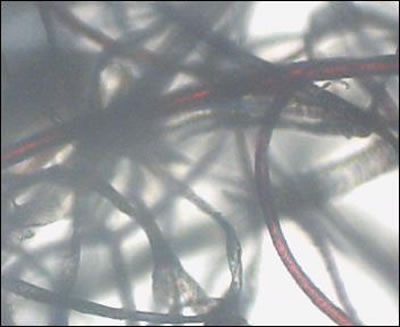

Agrobacterium

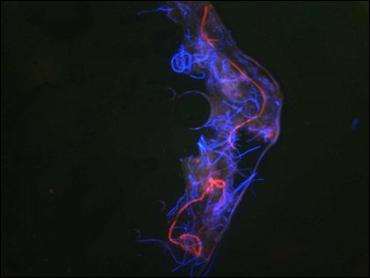

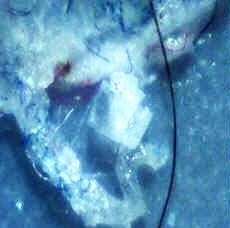
lip lesion
on 3 yr old boy
on 3 yr old boy